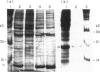
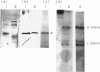
Abstract
Naturally occurring autoantibodies against cytokines exist in the sera of patients with autoimmune diseases as well as in the sera of normal individuals. We report here that affinity-purified autoantibodies against human TNF-alpha from one rheumatoid arthritis (RA) patient inhibited the cytotoxic effect of TNF-alpha on the mouse fibrosarcoma cell line WEHI 164, by 50%. In an attempt to predict the autoantibodies' recognition site on TNF-alpha protein we screened a random nanopeptide phage library with the affinity-purified TNF-alpha autoantibodies. Among 63 random selected clones, 46 clones carried the sequence ASSLLASSP, NSSPYLNTK or PQSPGSSFP. Frequency analysis of the relative occurrence of the 20 amino acids in the nanopeptides displayed by 50 random bacteriophages picked before selection and 63 after selection to bind to TNF-alpha autoantibodies indicated that proline (P < 0.0003) and serine (P < 0.04) are involved in the binding of the autoantibodies to the phages. Furthermore, we demonstrated that three synthetic peptides (ASSLLASSP, NSSPYLNTK and PPLKPVIDE) displayed by the selected phages reduced the binding of the autoantibodies to TNF-alpha protein by 50%. Interestingly, the sera of mice (BALB/c) immunized with phages displaying ASSLLASSP and NSSPYLNTK peptide showed an anti-TNF-alpha response as detected by ELISA. This response was not found in mice immunized with the wild type phage. Thus, the recombinant phages selected from the phage libraries could be used as carrier for immunization, and therefore as a tool for vaccine development. This work sets the stage for experiments designed to isolate ligands for protective antibodies.
Full text
PDF

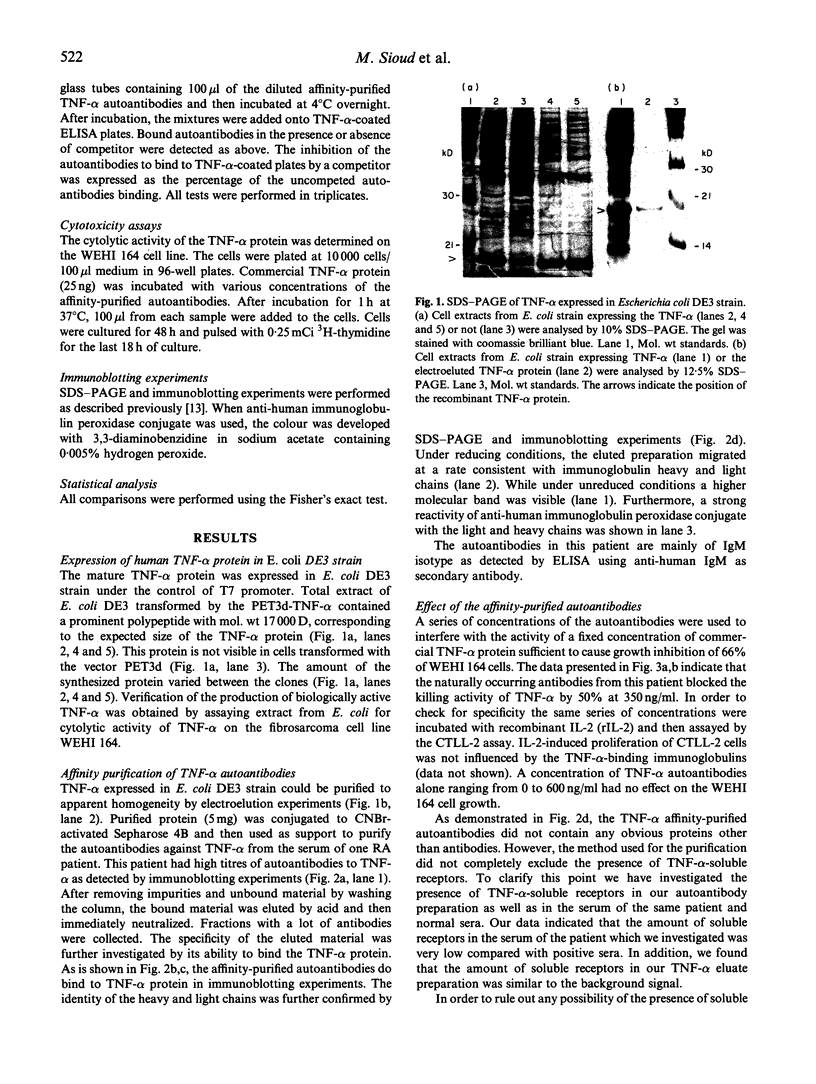

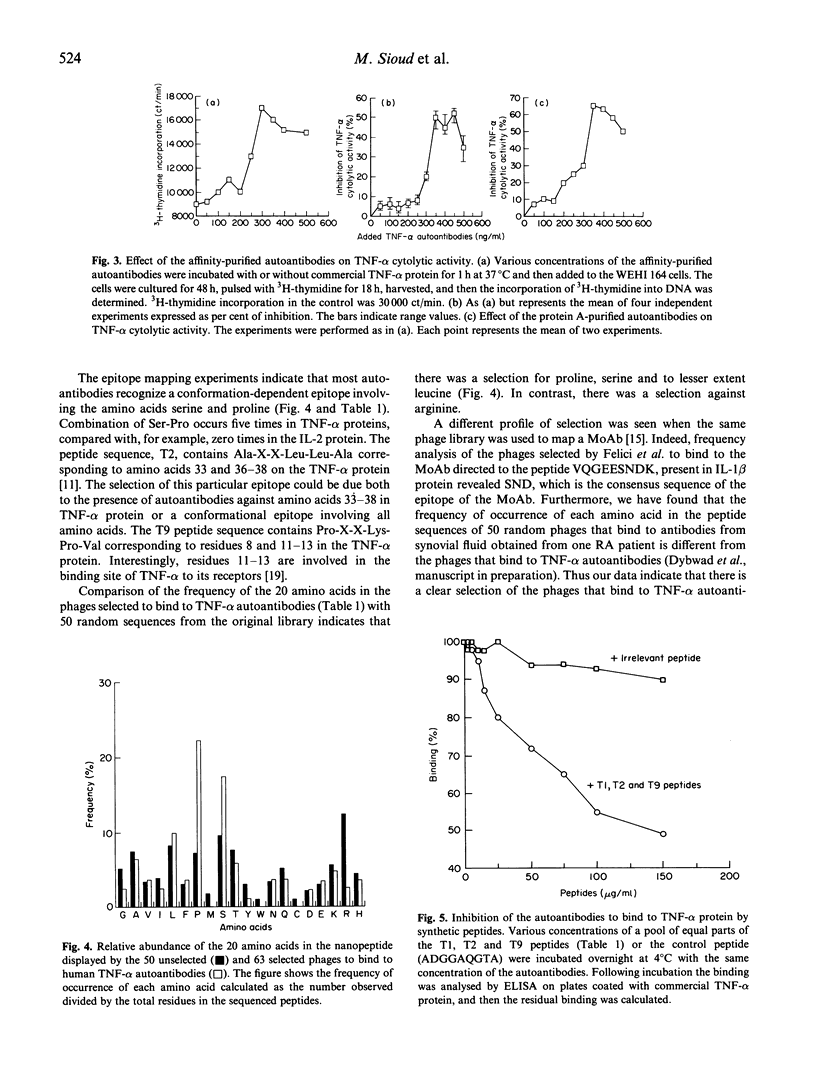

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Engelmann H., Maor Y., Brakebusch C., Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J Exp Med. 1992 Feb 1;175(2):323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Casali P., Notkins A. L. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu Rev Immunol. 1989;7:513–535. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- Deleuran B. W., Chu C. Q., Field M., Brennan F. M., Mitchell T., Feldmann M., Maini R. N. Localization of tumor necrosis factor receptors in the synovial tissue and cartilage-pannus junction in patients with rheumatoid arthritis. Implications for local actions of tumor necrosis factor alpha. Arthritis Rheum. 1992 Oct;35(10):1170–1178. doi: 10.1002/art.1780351009. [DOI] [PubMed] [Google Scholar]
- Dybwad A., Førre O., Kjeldsen-Kragh J., Natvig J. B., Sioud M. Identification of new B cell epitopes in the sera of rheumatoid arthritis patients using a random nanopeptide phage library. Eur J Immunol. 1993 Dec;23(12):3189–3193. doi: 10.1002/eji.1830231222. [DOI] [PubMed] [Google Scholar]
- Eck M. J., Sprang S. R. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J Biol Chem. 1989 Oct 15;264(29):17595–17605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- Elliott M. J., Maini R. N., Feldmann M., Long-Fox A., Charles P., Katsikis P., Brennan F. M., Walker J., Bijl H., Ghrayeb J. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993 Dec;36(12):1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- Felici F., Castagnoli L., Musacchio A., Jappelli R., Cesareni G. Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J Mol Biol. 1991 Nov 20;222(2):301–310. doi: 10.1016/0022-2836(91)90213-p. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Madden K. B., Morris S. C., Holmes J. M., Boiani N., Katona I. M., Maliszewski C. R. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993 Aug 1;151(3):1235–1244. [PubMed] [Google Scholar]
- Fomsgaard A., Svenson M., Bendtzen K. Auto-antibodies to tumour necrosis factor alpha in healthy humans and patients with inflammatory diseases and gram-negative bacterial infections. Scand J Immunol. 1989 Aug;30(2):219–223. doi: 10.1111/j.1365-3083.1989.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Griffiths A. D., Malmqvist M., Marks J. D., Bye J. M., Embleton M. J., McCafferty J., Baier M., Holliger K. P., Gorick B. D., Hughes-Jones N. C. Human anti-self antibodies with high specificity from phage display libraries. EMBO J. 1993 Feb;12(2):725–734. doi: 10.1002/j.1460-2075.1993.tb05706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffes E. W., 3rd, Ininns E. K., Schmitz K. L., Yamamoto R. S., Dett C. A., Granger G. A. The presence of antibodies to lymphotoxin and tumor necrosis factor in normal serum. Arthritis Rheum. 1989 Sep;32(9):1148–1152. doi: 10.1002/anr.1780320914. [DOI] [PubMed] [Google Scholar]
- Keffer J., Probert L., Cazlaris H., Georgopoulos S., Kaslaris E., Kioussis D., Kollias G. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991 Dec;10(13):4025–4031. doi: 10.1002/j.1460-2075.1991.tb04978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Davis L. S., Cush J. J., Oppenheimer-Marks N. The role of cytokines in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1989;11(2):123–162. doi: 10.1007/BF00197186. [DOI] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Schattner A. Lymphokines in autoimmunity--a critical review. Clin Immunol Immunopathol. 1994 Mar;70(3):177–189. doi: 10.1006/clin.1994.1027. [DOI] [PubMed] [Google Scholar]
- Sioud M., Drlica K. Prevention of human immunodeficiency virus type 1 integrase expression in Escherichia coli by a ribozyme. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7303–7307. doi: 10.1073/pnas.88.16.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Feldmann M., Maini R. N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz V. F., Lal A. A., McCutchan T. F. Immunogenicity and epitope mapping of foreign sequences via genetically engineered filamentous phage. J Biol Chem. 1988 Mar 25;263(9):4318–4322. [PubMed] [Google Scholar]