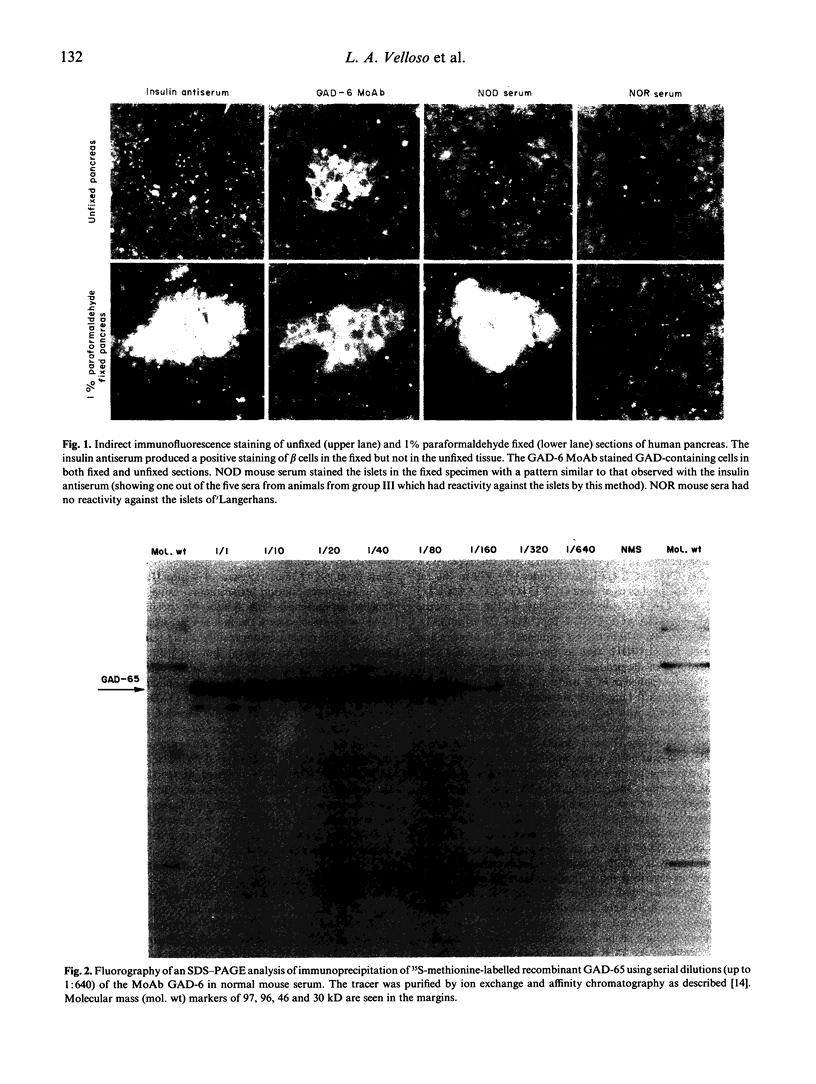
Abstract
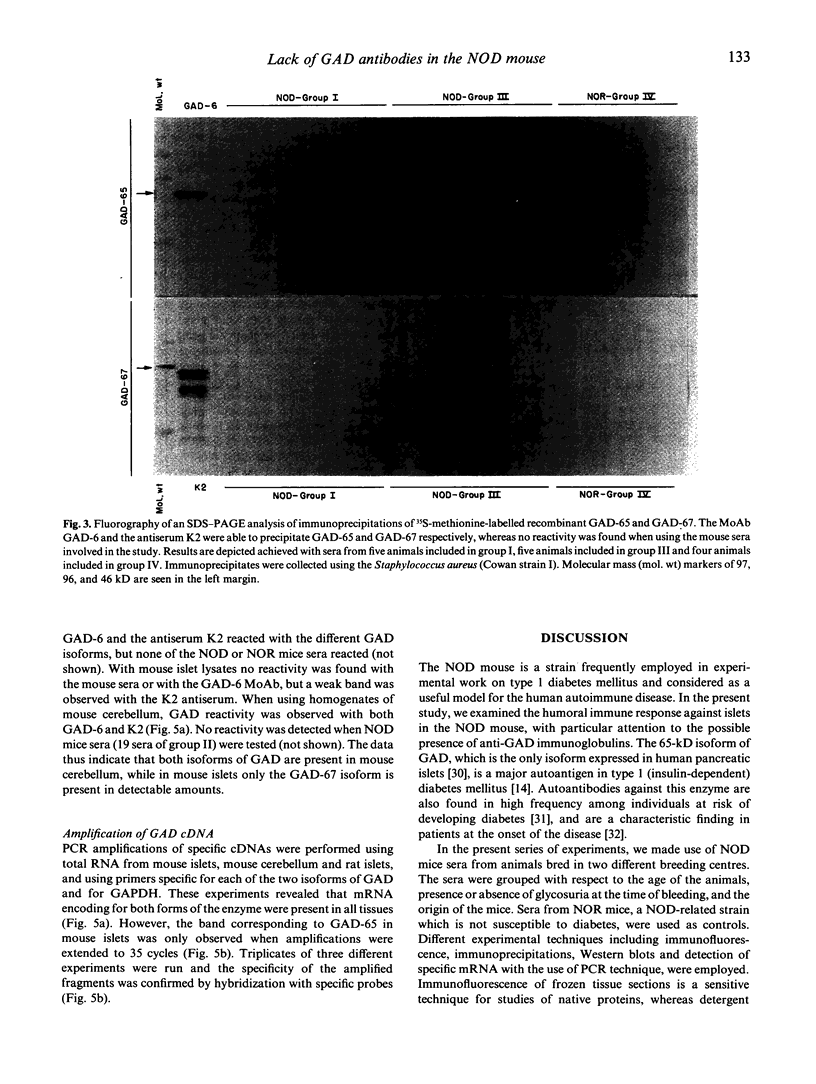
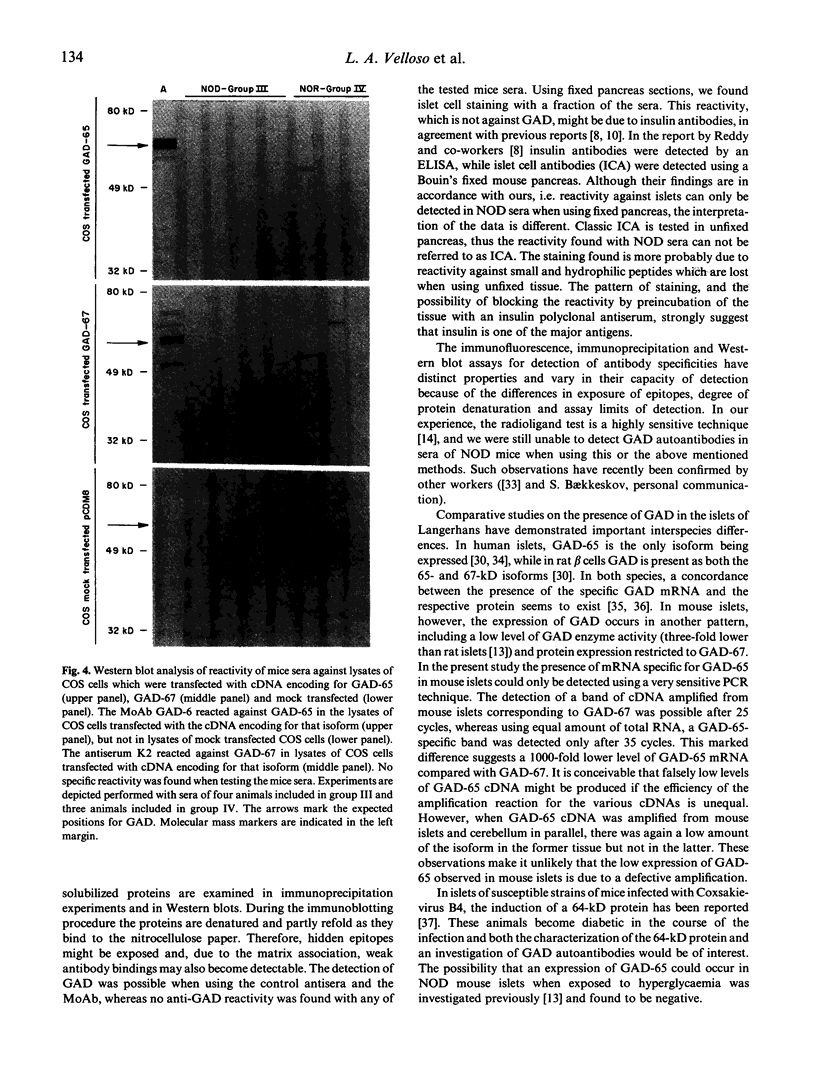
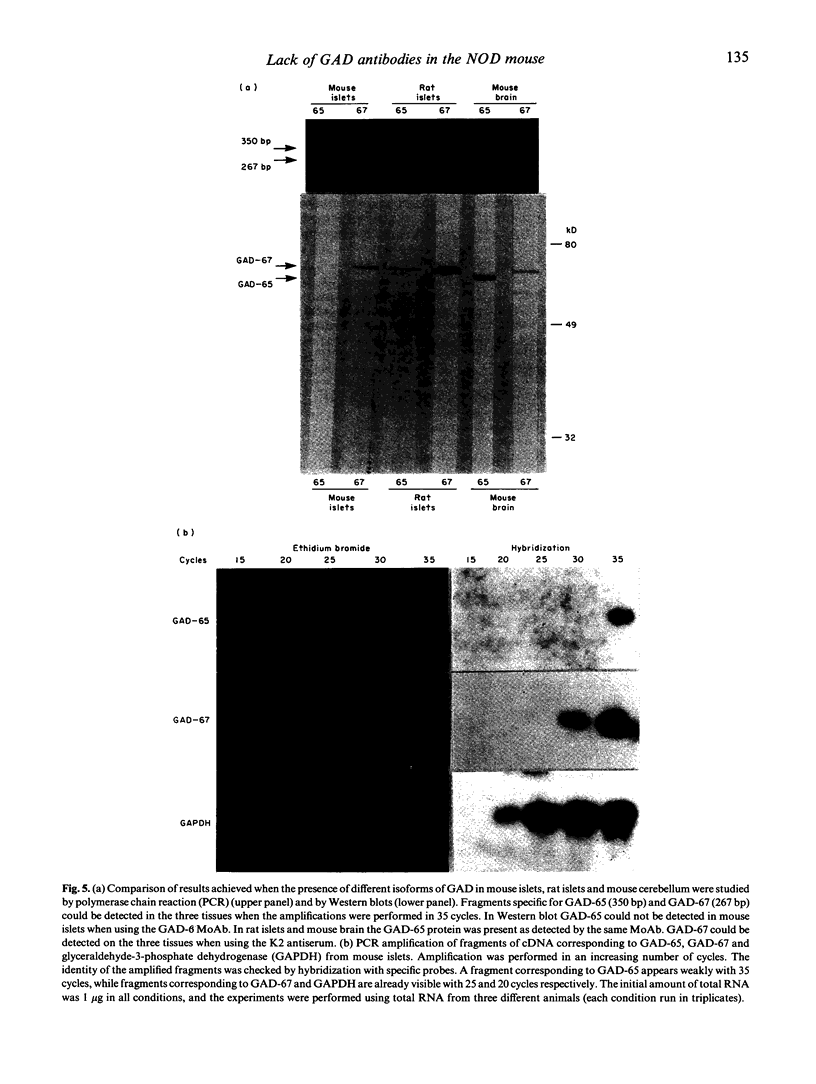
GAD is a major islet cell autoantigen in human type 1 diabetes mellitus. Autoantibodies are preferentially directed against the 65-kD isoform of the enzyme which is the only form expressed in human islets of Langerhans. The NOD mouse is a spontaneous model of type 1 diabetes, frequently employed in studies dealing with the immunopathogenesis of the disease. In the present study the reactivity of sera from 34 prediabetic and 15 diabetic NOD mice was tested against GAD protein present in islets of Langerhans and cerebellum, and against recombinant, semi-purified GAD-65 and GAD-67. A rabbit antiserum (K2) raised against GAD-67 could readily recognize the recombinant GAD-67 and the isoform present in rat and mouse islets and mouse brain. A MoAb (GAD-6) specific for the GAD-65 isoform reacted against the recombinant GAD-65 and the isoform present in rat islets and mouse brain, whereas no reactivity was observed when using mouse islets. However, when testing the NOD mice sera by immunohistochemistry, immunoprecipitation and Western blot, no reactivity against any of the isoforms of GAD could be detected. Using reverse transcription polymerase chain reaction (PCR), GAD-67 mRNA could be detected in mouse and rat islets and in mouse brain. GAD-65 mRNA could also be detected in rat islets and mouse brain, but apparently a much lower copy number is present in mouse islets. These findings stress important differences in the immune response occurring in the animal model NOD mouse compared with human type 1 diabetes, and emphasize that human and animal type 1 diabetes possibly represent the final outcome of several different etiological factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978 Jun;14(6):397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Kaufman D. L., Campbell L., Gibbs K. A., Shah S. C., Bu D. F., Erlander M. G., Tobin A. J., Maclaren N. K. Response of peripheral-blood mononuclear cells to glutamate decarboxylase in insulin-dependent diabetes. Lancet. 1992 Feb 22;339(8791):458–459. doi: 10.1016/0140-6736(92)91061-c. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K. Autoantibodies in nonobese diabetic mice immunoprecipitate 64,000-Mr islet antigen. Diabetes. 1988 Nov;37(11):1587–1590. doi: 10.2337/diab.37.11.1587. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Landin M., Kristensen J. K., Srikanta S., Bruining G. J., Mandrup-Poulsen T., de Beaufort C., Soeldner J. S., Eisenbarth G., Lindgren F. Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest. 1987 Mar;79(3):926–934. doi: 10.1172/JCI112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Ballagi-Pordány A., Ballagi-Pordány A., Funa K. Quantitative determination of mRNA phenotypes by the polymerase chain reaction. Anal Biochem. 1991 Jul;196(1):89–94. doi: 10.1016/0003-2697(91)90122-a. [DOI] [PubMed] [Google Scholar]
- Björk E., Kämpe O., Karlsson F. A., Pipeleers D. G., Andersson A., Hellerström C., Eizirik D. L. Glucose regulation of the autoantigen GAD65 in human pancreatic islets. J Clin Endocrinol Metab. 1992 Dec;75(6):1574–1576. doi: 10.1210/jcem.75.6.1464667. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu D. F., Erlander M. G., Hitz B. C., Tillakaratne N. J., Kaufman D. L., Wagner-McPherson C. B., Evans G. A., Tobin A. J. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christianson S. W., Shultz L. D., Leiter E. H. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993 Jan;42(1):44–55. doi: 10.2337/diab.42.1.44. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler G. S., Basile A. A., Dieffenbach C. W. Analysis of gene expression: use of oligonucleotide primers for glyceraldehyde-3-phosphate dehydrogenase. PCR Methods Appl. 1992 May;1(4):283–285. doi: 10.1101/gr.1.4.283. [DOI] [PubMed] [Google Scholar]
- Erlander M. G., Tillakaratne N. J., Feldblum S., Patel N., Tobin A. J. Two genes encode distinct glutamate decarboxylases. Neuron. 1991 Jul;7(1):91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Gaskins H. R., Prochazka M., Hamaguchi K., Serreze D. V., Leiter E. H. Beta cell expression of endogenous xenotropic retrovirus distinguishes diabetes-susceptible NOD/Lt from resistant NON/Lt mice. J Clin Invest. 1992 Dec;90(6):2220–2227. doi: 10.1172/JCI116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerling I., Nejman C., Chatterjee N. K. Effect of coxsackievirus B4 infection in mice on expression of 64,000-Mr autoantigen and glucose sensitivity of islets before development of hyperglycemia. Diabetes. 1988 Oct;37(10):1419–1425. doi: 10.2337/diab.37.10.1419. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I., Chang Y. C., Schwob J. E. Monoclonal antibodies to glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8808–8812. doi: 10.1073/pnas.83.22.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins K., McDuffie M. Acceleration of diabetes in young NOD mice with a CD4+ islet-specific T cell clone. Science. 1990 Sep 21;249(4975):1433–1436. doi: 10.1126/science.2205920. [DOI] [PubMed] [Google Scholar]
- Karlsen A. E., Hagopian W. A., Grubin C. E., Dube S., Disteche C. M., Adler D. A., Bärmeier H., Mathewes S., Grant F. J., Foster D. Cloning and primary structure of a human islet isoform of glutamic acid decarboxylase from chromosome 10. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8337–8341. doi: 10.1073/pnas.88.19.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katarova Z., Szabo G., Mugnaini E., Greenspan R. J. Molecular Identification of the 62 kd Form of Glutamic Acid Decarboxylase from the Mouse. Eur J Neurosci. 1990;2(3):190–202. doi: 10.1111/j.1460-9568.1990.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Kaufman D. L., Clare-Salzler M., Tian J., Forsthuber T., Ting G. S., Robinson P., Atkinson M. A., Sercarz E. E., Tobin A. J., Lehmann P. V. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature. 1993 Nov 4;366(6450):69–72. doi: 10.1038/366069a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Richter W., Aanstoot H. J., Shi Y., Fu Q., Rajotte R., Warnock G., Baekkeskov S. Differential expression of GAD65 and GAD67 in human, rat, and mouse pancreatic islets. Diabetes. 1993 Dec;42(12):1799–1808. doi: 10.2337/diab.42.12.1799. [DOI] [PubMed] [Google Scholar]
- Kämpe O., Andersson A., Björk E., Hallberg A., Karlsson F. A. High-glucose stimulation of 64,000-Mr islet cell autoantigen expression. Diabetes. 1989 Oct;38(10):1326–1328. doi: 10.2337/diab.38.10.1326. [DOI] [PubMed] [Google Scholar]
- Makino S., Kunimoto K., Muraoka Y., Mizushima Y., Katagiri K., Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980 Jan;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- Michel C., Boitard C., Bach J. F. Insulin autoantibodies in non-obese diabetic (NOD) mice. Clin Exp Immunol. 1989 Mar;75(3):457–460. [PMC free article] [PubMed] [Google Scholar]
- Michelsen B. K., Petersen J. S., Boel E., Møldrup A., Dyrberg T., Madsen O. D. Cloning, characterization, and autoimmune recognition of rat islet glutamic acid decarboxylase in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8754–8758. doi: 10.1073/pnas.88.19.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki A., Hanafusa T., Yamada K., Miyagawa J., Fujino-Kurihara H., Nakajima H., Nonaka K., Tarui S. Predominance of T lymphocytes in pancreatic islets and spleen of pre-diabetic non-obese diabetic (NOD) mice: a longitudinal study. Clin Exp Immunol. 1985 Jun;60(3):622–630. [PMC free article] [PubMed] [Google Scholar]
- Petersen J. S., Russel S., Marshall M. O., Kofod H., Buschard K., Cambon N., Karlsen A. E., Boel E., Hagopian W. A., Hejnaes K. R. Differential expression of glutamic acid decarboxylase in rat and human islets. Diabetes. 1993 Mar;42(3):484–495. doi: 10.2337/diab.42.3.484. [DOI] [PubMed] [Google Scholar]
- Pontesilli O., Carotenuto P., Gazda L. S., Pratt P. F., Prowse S. J. Circulating lymphocyte populations and autoantibodies in non-obese diabetic (NOD) mice: a longitudinal study. Clin Exp Immunol. 1987 Oct;70(1):84–93. [PMC free article] [PubMed] [Google Scholar]
- Pozzilli P., Signore A., Williams A. J., Beales P. E. NOD mouse colonies around the world--recent facts and figures. Immunol Today. 1993 May;14(5):193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- Prochazka M., Serreze D. V., Frankel W. N., Leiter E. H. NOR/Lt mice: MHC-matched diabetes-resistant control strain for NOD mice. Diabetes. 1992 Jan;41(1):98–106. doi: 10.2337/diab.41.1.98. [DOI] [PubMed] [Google Scholar]
- Reddy S., Bibby N. J., Elliott R. B. Ontogeny of islet cell antibodies, insulin autoantibodies and insulitis in the non-obese diabetic mouse. Diabetologia. 1988 May;31(5):322–328. doi: 10.1007/BF00277415. [DOI] [PubMed] [Google Scholar]
- Signore A., Pozzilli P., Gale E. A., Andreani D., Beverley P. C. The natural history of lymphocyte subsets infiltrating the pancreas of NOD mice. Diabetologia. 1989 May;32(5):282–289. doi: 10.1007/BF00265543. [DOI] [PubMed] [Google Scholar]
- Tisch R., Yang X. D., Singer S. M., Liblau R. S., Fugger L., McDevitt H. O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993 Nov 4;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- Velloso L. A., Kämpe O., Eizirik D. L., Hallberg A., Andersson A., Karlsson F. A. Human autoantibodies react with glutamic acid decarboxylase antigen in human and rat but not in mouse pancreatic islets. Diabetologia. 1993 Jan;36(1):39–46. doi: 10.1007/BF00399091. [DOI] [PubMed] [Google Scholar]
- Velloso L. A., Kämpe O., Hallberg A., Christmanson L., Betsholtz C., Karlsson F. A. Demonstration of GAD-65 as the main immunogenic isoform of glutamate decarboxylase in type 1 diabetes and determination of autoantibodies using a radioligand produced by eukaryotic expression. J Clin Invest. 1993 May;91(5):2084–2090. doi: 10.1172/JCI116431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilberz S., Partke H. J., Dagnaes-Hansen F., Herberg L. Persistent MHV (mouse hepatitis virus) infection reduces the incidence of diabetes mellitus in non-obese diabetic mice. Diabetologia. 1991 Jan;34(1):2–5. doi: 10.1007/BF00404016. [DOI] [PubMed] [Google Scholar]
- Winqvist O., Karlsson F. A., Kämpe O. 21-Hydroxylase, a major autoantigen in idiopathic Addison's disease. Lancet. 1992 Jun 27;339(8809):1559–1562. doi: 10.1016/0140-6736(92)91829-w. [DOI] [PubMed] [Google Scholar]