Abstract
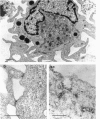
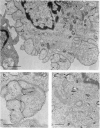
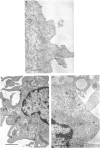
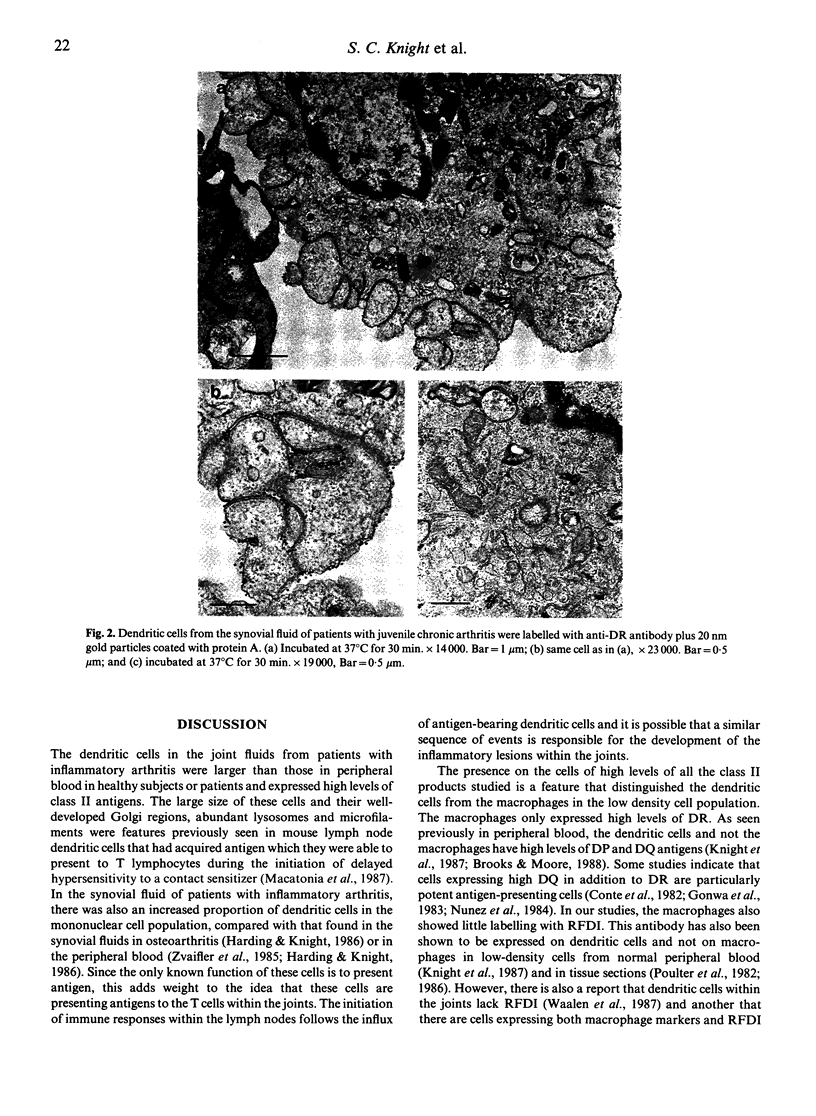
Dendritic cells were enriched from synovial fluids (SF) of patients with inflammatory arthritis and studied by immunogold labelling and electron microscopy for expression of histocompatability antigens of the HLA-D locus. Dendritic cells from SF were larger than most of these from peripheral blood with a more extensive Golgi region and more lysosomes and microfilaments. Class II histocompatability antigens HLA-DR, -DP, -DQ and that labelled by the antibody RFDI were abundant on the dendritic cells. The macrophages in the enriched cells showed labelling for DR but little labelling with the other antibodies. DR, DP and RFDI were often concentrated at areas of contact between dendritic and other cells (other dendritic cells, macrophages or lymphocytes). On incubating labelled cells at 37 degrees C for 30 min many macrophages lost their DR label but dendritic cells always retained some surface label. Some gold labelling DR and DP was found in characteristic channels between the veils and became internalized in membrane-bound structures. A small proportion of the RFDI label internalized in areas resembling coated pits. Less DQ label internalized and appeared on vesicles inside vacuoles. Material bound to different class II molecules may thus be internalized or processed differently by dendritic cells. The presence in inflammatory lesions of large activated dendritic cells with high expression of class II antigens suggests that these cells could be presenting antigen to lymphocytes within the joints.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M. Lymphoid dendritic cells. Immunology. 1987 Oct;62(2):161–170. [PMC free article] [PubMed] [Google Scholar]
- Brooks C. F., Moore M. Differential MHC class II expression on human peripheral blood monocytes and dendritic cells. Immunology. 1988 Feb;63(2):303–311. [PMC free article] [PubMed] [Google Scholar]
- Corte G., Moretta A., Cosulich M. E., Ramarli D., Bargellesi A. A monoclonal anti-DC1 antibody selectivity inhibits the generation of effector T cells mediating specific cytolytic activity. J Exp Med. 1982 Nov 1;156(5):1539–1544. doi: 10.1084/jem.156.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H. G., Gignac S. M., Brenner M. K., Coustan-Smith E., Janossy G., Hoffbrand A. V. Differential expression of MHC class II antigens in chronic B-cell disorders. Clin Exp Immunol. 1988 Feb;71(2):217–223. [PMC free article] [PubMed] [Google Scholar]
- Gonwa T. A., Picker L. J., Raff H. V., Goyert S. M., Silver J., Stobo J. D. Antigen-presenting capabilities of human monocytes correlates with their expression of HLA-DS, an Ia determinant distinct from HLA-DR. J Immunol. 1983 Feb;130(2):706–711. [PubMed] [Google Scholar]
- Hanau D., Fabre M., Schmitt D. A., Garaud J. C., Pauly G., Tongio M. M., Mayer S., Cazenave J. P. Human epidermal Langerhans cells cointernalize by receptor-mediated endocytosis "nonclassical" major histocompatibility complex class I molecules (T6 antigens) and class II molecules (HLA-DR antigens). Proc Natl Acad Sci U S A. 1987 May;84(9):2901–2905. doi: 10.1073/pnas.84.9.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding B., Knight S. C. The distribution of dendritic cells in the synovial fluids of patients with arthritis. Clin Exp Immunol. 1986 Mar;63(3):594–600. [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Antigen processing and intracellular Ia. Possible roles of endocytosis and protein synthesis in Ia function. J Immunol. 1989 Jan 1;142(1):12–19. [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Chan J., Bryant A., Bedford P. A., Bateman C. Induction of autoimmunity with dendritic cells: studies on thyroiditis in mice. Clin Immunol Immunopathol. 1988 Sep;48(3):277–289. doi: 10.1016/0090-1229(88)90021-9. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Mertin J., Stackpoole A., Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6032–6035. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. C. The autologous mixed lymphocyte reaction and dendritic cells. Br J Rheumatol. 1987 Apr;26(2):85–88. doi: 10.1093/rheumatology/26.2.85. [DOI] [PubMed] [Google Scholar]
- Lechler R. I., Batchelor J. R. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982 Jan 1;155(1):31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Knight S. C., Edwards A. J., Griffiths S., Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J Exp Med. 1987 Dec 1;166(6):1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S., Knight S. C. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J Gen Virol. 1987 Apr;68(Pt 4):1177–1181. doi: 10.1099/0022-1317-68-4-1177. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G. Histochemical discrimination of HLA-DR positive cell populations in the normal and arthritic synovial lining. Clin Exp Immunol. 1982 May;48(2):381–388. [PMC free article] [PubMed] [Google Scholar]
- Salisbury A. K., Duke O., Poulter L. W. Macrophage-like cells of the pannus area in rheumatoid arthritic joints. Scand J Rheumatol. 1987;16(4):263–272. doi: 10.3109/03009748709102927. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Waalen K., Førre O., Pahle J., Natvig J. B., Burmester G. R. Characteristics of human rheumatoid synovial and normal blood dendritic cells. Retention of class II major histocompatibility complex antigens and accessory function after short-term culture. Scand J Immunol. 1987 Nov;26(5):525–533. doi: 10.1111/j.1365-3083.1987.tb02286.x. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J., Steinman R. M., Kaplan G., Lau L. L., Rivelis M. Identification of immunostimulatory dendritic cells in the synovial effusions of patients with rheumatoid arthritis. J Clin Invest. 1985 Aug;76(2):789–800. doi: 10.1172/JCI112036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vere Tyndall A., Knight S. C., Edwards A. J., Clarke J. B. Veiled (dendritic) cells in synovial fluid. Lancet. 1983 Feb 26;1(8322):472–473. doi: 10.1016/s0140-6736(83)91468-x. [DOI] [PubMed] [Google Scholar]