Abstract
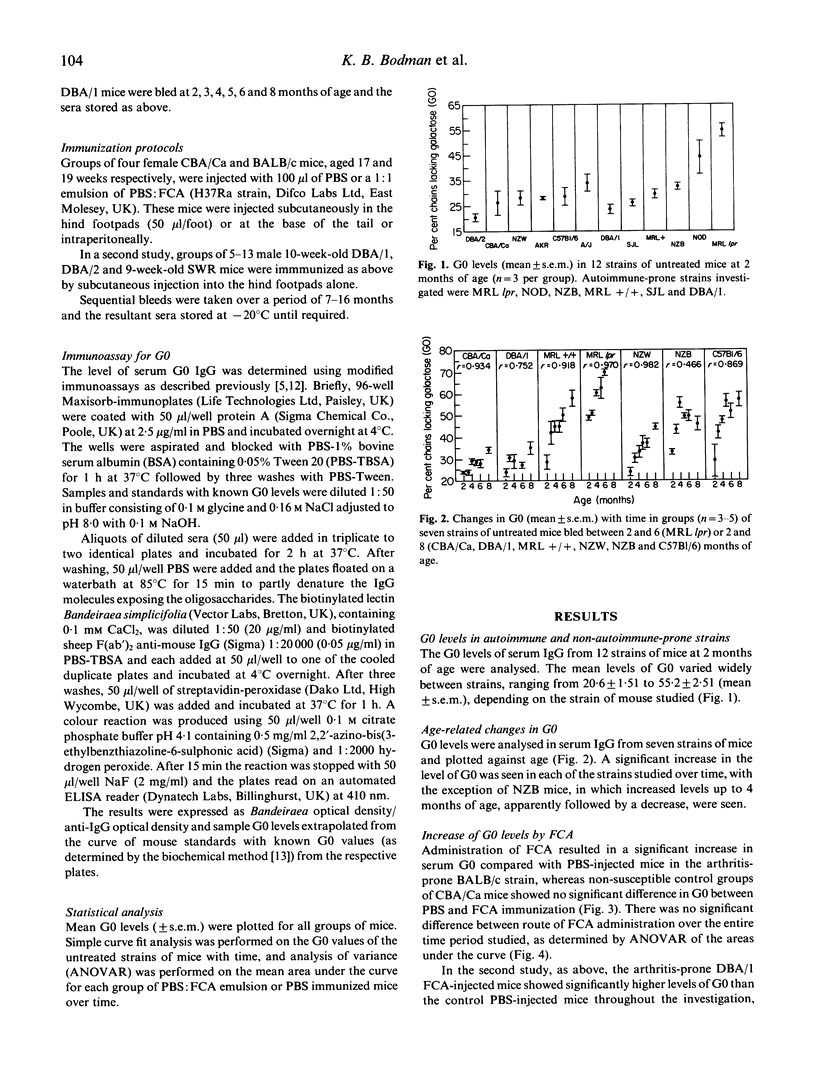
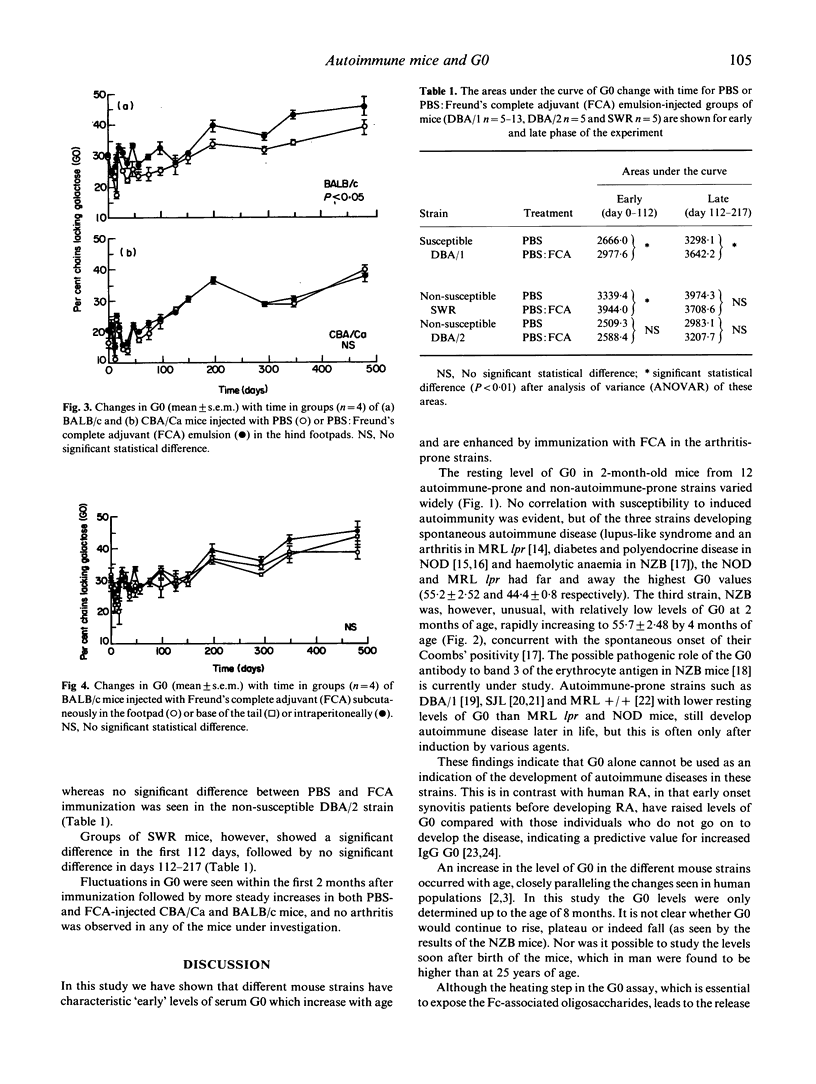
The relationship between increased levels of IgG oligosaccharide chains lacking galactose (G0) and the development of rheumatoid arthritis is unclear. In order to further our understanding of the observed correlation between raised serum G0 and arthritis, we have studied G0 levels in arthritis-prone and non-susceptible (i.e. non-arthritis-prone) mice and the effects on G0 of mycobacterial antigens, which have been postulated to play a role in the early events leading to the development of arthritis. We have shown that different age-matched mouse strains have characteristic 'resting' levels of G0 which (in six out of seven strains of mice) increase with age. We have also shown that these increases can be enhanced by immunization of arthritis-prone strains of mice with an adjuvant containing mycobacteria (Freund's complete adjuvant (FCA)), suggesting that deflects in the ability to regulate these G0 changes may be related to susceptibility to arthritis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker R. N., de Sá Oliveira G. G., Elson C. J., Lydyard P. M. Pathogenic autoantibodies in the NZB mouse are specific for erythrocyte band 3 protein. Eur J Immunol. 1993 Jul;23(7):1723–1726. doi: 10.1002/eji.1830230750. [DOI] [PubMed] [Google Scholar]
- Bernard N. F., Ertug F., Margolese H. High incidence of thyroiditis and anti-thyroid autoantibodies in NOD mice. Diabetes. 1992 Jan;41(1):40–46. doi: 10.2337/diab.41.1.40. [DOI] [PubMed] [Google Scholar]
- Bond A., Cooke A., Hay F. C. Glycosylation of IgG, immune complexes and IgG subclasses in the MRL-lpr/lpr mouse model of rheumatoid arthritis. Eur J Immunol. 1990 Oct;20(10):2229–2233. doi: 10.1002/eji.1830201011. [DOI] [PubMed] [Google Scholar]
- Chronopoulou E., Carayanniotis G. Identification of a thyroiditogenic sequence within the thyroglobulin molecule. J Immunol. 1992 Aug 1;149(3):1039–1044. [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Dubé R., Rook G. A., Steele J., Brealey R., Dwek R., Rademacher T., Lennard-Jones J. Agalactosyl IgG in inflammatory bowel disease: correlation with C-reactive protein. Gut. 1990 Apr;31(4):431–434. doi: 10.1136/gut.31.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler H. M., Rook G. A., Johnson N. M., McFadden J. Mycobacterium tuberculosis DNA in tissue affected by sarcoidosis. BMJ. 1993 Feb 27;306(6877):546–549. doi: 10.1136/bmj.306.6877.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filley E., Andreoli A., Steele J., Waters M., Wagner D., Nelson D., Tung K., Rademacher T., Dwek R., Rook G. A. A transient rise in agalactosyl IgG correlating with free interleukin 2 receptors, during episodes of erythema nodosum leprosum. Clin Exp Immunol. 1989 Jun;76(3):343–347. [PMC free article] [PubMed] [Google Scholar]
- HOLMES M. C., BURNET F. M. THE NATURAL HISTORY OF AUTOIMMUNE DISEASE IN NZB MICE. A COMPARISON WITH THE PATTERN OF HUMAN AUTOIMMUNE MANIFESTATIONS. Ann Intern Med. 1963 Sep;59:265–276. doi: 10.7326/0003-4819-59-3-265. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Andersson M., Goldschmidt T. J., Gustafsson K., Jansson L., Mo J. A. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990 Dec;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Andersson M., Jonsson R. Genetic, hormonal and behavioural influence on spontaneously developing arthritis in normal mice. Clin Exp Immunol. 1992 Jun;88(3):467–472. doi: 10.1111/j.1365-2249.1992.tb06473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikutani H., Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- Knight B., Katz D. R., Isenberg D. A., Ibrahim M. A., Le Page S., Hutchings P., Schwartz R. S., Cooke A. Induction of adjuvant arthritis in mice. Clin Exp Immunol. 1992 Dec;90(3):459–465. doi: 10.1111/j.1365-2249.1992.tb05868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Lider O., al-Sabbagh A., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. V. Hierarchy of suppression by myelin basic protein from different species. J Neuroimmunol. 1992 Aug;39(3):243–250. doi: 10.1016/0165-5728(92)90258-m. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Hamako J., Nose M., Titani K. Structural changes in the oligosaccharide chains of IgG in autoimmune MRL/Mp-lpr/lpr mice. J Immunol. 1990 Sep 15;145(6):1794–1798. [PubMed] [Google Scholar]
- Nordling C., Karlsson-Parra A., Jansson L., Holmdahl R., Klareskog L. Characterization of a spontaneously occurring arthritis in male DBA/1 mice. Arthritis Rheum. 1992 Jun;35(6):717–722. doi: 10.1002/art.1780350619. [DOI] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- Parekh R., Roitt I., Isenberg D., Dwek R., Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988 May 1;167(5):1731–1736. doi: 10.1084/jem.167.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A., Isenberg D., Rook G., Axford J. S., Roitt I. The role of IgG glycoforms in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1988;10(2-3):231–249. doi: 10.1007/BF01857227. [DOI] [PubMed] [Google Scholar]
- Rook G. A., al Attiyah R., Filley E. New insights into the immunopathology of tuberculosis. Pathobiology. 1991;59(3):148–152. doi: 10.1159/000163633. [DOI] [PubMed] [Google Scholar]
- Rook G., Thompson S., Buckley M., Elson C., Brealey R., Lambert C., White T., Rademacher T. The role of oil and agalactosyl IgG in the induction of arthritis in rodent models. Eur J Immunol. 1991 Apr;21(4):1027–1032. doi: 10.1002/eji.1830210425. [DOI] [PubMed] [Google Scholar]
- Shirai A., Holmes K., Klinman D. Detection and quantitation of cells secreting IL-6 under physiologic conditions in BALB/c mice. J Immunol. 1993 Feb 1;150(3):793–799. [PubMed] [Google Scholar]
- Sumar N., Bodman K. B., Rademacher T. W., Dwek R. A., Williams P., Parekh R. B., Edge J., Rook G. A., Isenberg D. A., Hay F. C. Analysis of glycosylation changes in IgG using lectins. J Immunol Methods. 1990 Jul 20;131(1):127–136. doi: 10.1016/0022-1759(90)90242-n. [DOI] [PubMed] [Google Scholar]
- Tang B., Matsuda T., Akira S., Nagata N., Ikehara S., Hirano T., Kishimoto T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991 Mar;3(3):273–278. doi: 10.1093/intimm/3.3.273. [DOI] [PubMed] [Google Scholar]
- Thompson S. J., Hitsumoto Y., Zhang Y. W., Rook G. A., Elson C. J. Agalactosyl IgG in pristane-induced arthritis. Pregnancy affects the incidence and severity of arthritis and the glycosylation status of IgG. Clin Exp Immunol. 1992 Sep;89(3):434–438. doi: 10.1111/j.1365-2249.1992.tb06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley P. H. Collagen-induced arthritis in the mouse. Methods Enzymol. 1988;162:361–373. doi: 10.1016/0076-6879(88)62091-x. [DOI] [PubMed] [Google Scholar]
- Young A., Sumar N., Bodman K., Goyal S., Sinclair H., Roitt I., Isenberg D. Agalactosyl IgG: an aid to differential diagnosis in early synovitis. Arthritis Rheum. 1991 Nov;34(11):1425–1429. doi: 10.1002/art.1780341113. [DOI] [PubMed] [Google Scholar]


