Abstract
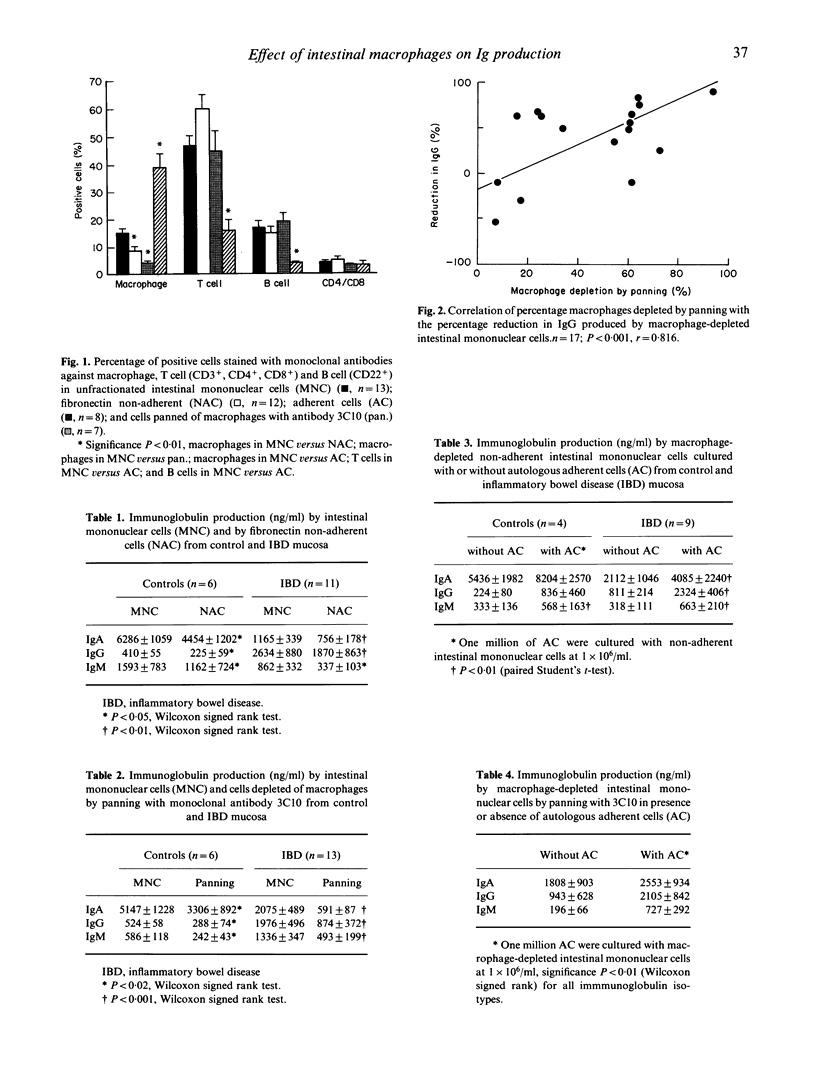
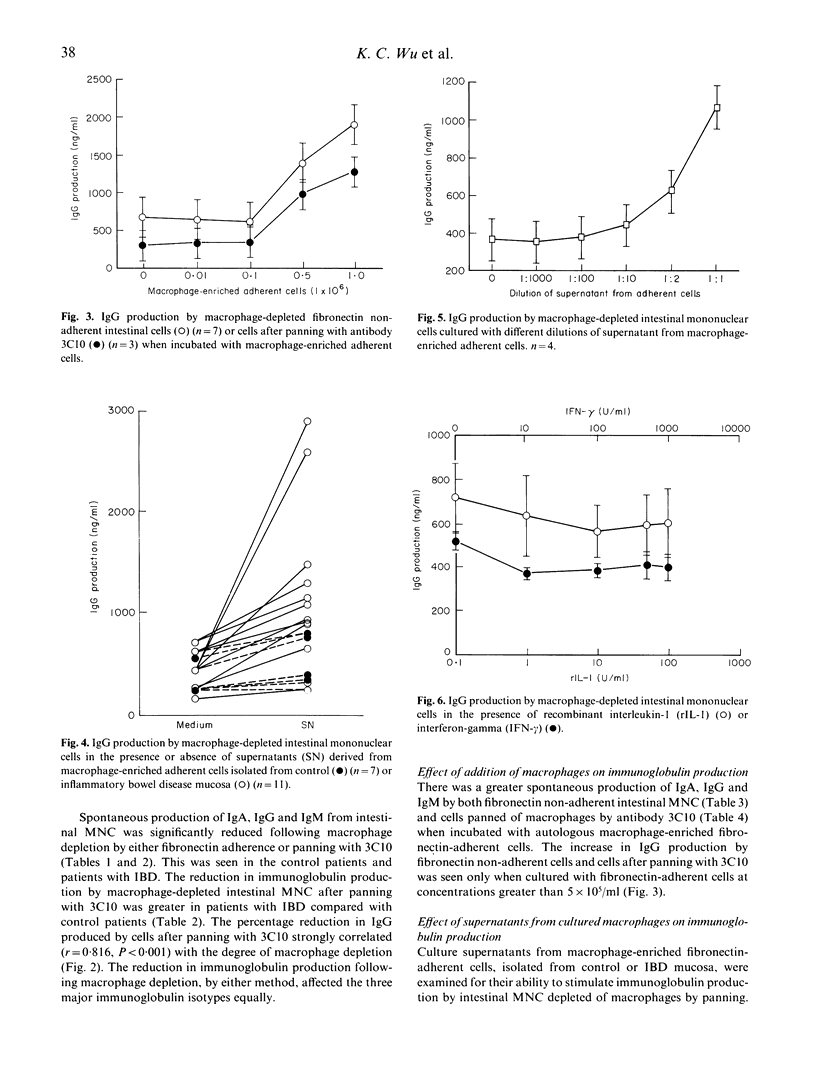
The effect of macrophages on spontaneous immunoglobulin production by isolated human intestinal mononuclear cells (MNC) is unknown. Depletion of macrophages by adherence to fibronectin or by panning with macrophage-specific monoclonal antibody 3C10 lead to a significant reduction in IgA. IgG and IgM production by intestinal MNC from both normal (n = 10) and inflammatory bowel disease (IBD) (n = 13) mucosa. The reduction in immunoglobulin produced by macrophage-depleted intestinal MNC was greater in IBD patients than in normal controls. There was a significant correlation (r = 0.816, P less than 0.001) between the percentage of macrophages depleted by panning with 3C10 and the reduction in IgG produced by macrophage-depleted intestinal MNC. Addition of either fibronectin-adherent cells or the supernatant from these macrophage-enriched cells enhanced immunoglobulin production in a dose-dependent fashion. A greater increase in IgG production by macrophage-depleted cells was seen when cultured with supernatant from inflamed IBD mucosal cells, compared with that from normal mucosal cells. The soluble factor(s) responsible in the supernatant was acid and heat susceptible but was not affected by freezing and thawing. Addition of recombinant human interleukin-1 beta or human interferon-gamma to cell cultures did not influence immunoglobulin production. Thus, human intestinal macrophages enhance spontaneous immunoglobulin production by isolated intestinal MNC by secreting soluble factor(s) which remain to be fully characterized.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett S. J. Regulatory interactions between macrophages and T cells in Mycobacterium lepraemurium-specific T-cell activation. Cell Immunol. 1987 Dec;110(2):379–390. doi: 10.1016/0008-8749(87)90130-4. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens J. L., Stevens E. A. Immunoglobulin production in cultures of pokeweed mitogen stimulated human peripheral blood mononuclear cells requires interaction of interleukin 2 with the B cells. Cell Immunol. 1986 Mar;98(1):1–7. doi: 10.1016/0008-8749(86)90261-3. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Doe W. F., Dorsman B. Chronic inflammatory bowel disease--increased plasminogen activator secretion by mononuclear phagocytes. Clin Exp Immunol. 1982 Apr;48(1):256–260. [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Machelski E., Weiserbs D. B. T cell-B cell regulation in the intestinal lamina propria in Crohn's disease. Gastroenterology. 1985 Aug;89(2):321–327. doi: 10.1016/0016-5085(85)90332-4. [DOI] [PubMed] [Google Scholar]
- Inaba K., Nakano K., Muramatsu S. Cellular synergy in the manifestation of accessory cell activity for in vitro antibody response. J Immunol. 1981 Aug;127(2):452–461. [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Van Voorhis W. C., Muramatsu S. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6041–6045. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Immunoregulatory function of lamina propria T cells in Crohn's disease. Gastroenterology. 1985 May;88(5 Pt 1):1143–1150. doi: 10.1016/s0016-5085(85)80073-1. [DOI] [PubMed] [Google Scholar]
- Kennedy M. S., Stobo J. D., Goldyne M. E. In vitro synthesis of prostaglandins and related lipids by populations of human peripheral blood mononuclear cells. Prostaglandins. 1980 Jul;20(1):135–145. doi: 10.1016/0090-6980(80)90013-1. [DOI] [PubMed] [Google Scholar]
- Kujawa M., Sawicki W., Kucharczyk K., Szymańska K. Macrophages of the intestinal wall: their role in the phagocytosis of migrating cells. Acta Med Pol. 1977;18(4):335–336. [PubMed] [Google Scholar]
- Lin H. S., Gordon S. Secretion of plasminogen activator by bone marrow-derived mononuclear phagocytes and its enhancement by colony-stimulating factor. J Exp Med. 1979 Aug 1;150(2):231–245. doi: 10.1084/jem.150.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott R. P., Nash G. S., Bertovich M. J., Seiden M. V., Bragdon M. J., Beale M. G. Alterations of IgM, IgG, and IgA Synthesis and secretion by peripheral blood and intestinal mononuclear cells from patients with ulcerative colitis and Crohn's disease. Gastroenterology. 1981 Nov;81(5):844–852. [PubMed] [Google Scholar]
- Maehara N., Ho M. Cellular origin of interferon induced by bacterial lipopolysaccharide. Infect Immun. 1977 Jan;15(1):78–83. doi: 10.1128/iai.15.1.78-83.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Wu K. C., Jewell D. P. Characterization of antigen-presenting activity of intestinal mononuclear cells isolated from normal and inflammatory bowel disease colon and ileum. Immunology. 1988 Dec;65(4):543–549. [PMC free article] [PubMed] [Google Scholar]
- Murray P. D., McKenzie D. T., Swain S. L., Kagnoff M. F. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987 Oct 15;139(8):2669–2674. [PubMed] [Google Scholar]
- Selby W. S., Poulter L. W., Hobbs S., Jewell D. P., Janossy G. Heterogeneity of HLA-DR-positive histiocytes in human intestinal lamina propria: a combined histochemical and immunohistological analysis. J Clin Pathol. 1983 Apr;36(4):379–384. doi: 10.1136/jcp.36.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Prostaglandins, macrophages, and immunity. J Immunol. 1980 Jul;125(1):1–5. [PubMed] [Google Scholar]
- Takemura R., Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984 Jan;246(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- Verspaget H. W., Peña A. S., Weterman I. T., Lamers C. B. Disordered regulation of the in vitro immunoglobulin synthesis by intestinal mononuclear cells in Crohn's disease. Gut. 1988 Apr;29(4):503–510. doi: 10.1136/gut.29.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter H. S., Cole F. S., Huffer L. M., Davidson C. B., Katz A. J., Edelson P. J. Isolation and characterization of resident macrophages from guinea pig and human intestine. Gastroenterology. 1983 Aug;85(2):358–363. [PubMed] [Google Scholar]
- Wu K. C., Mahida Y. R., Priddle J. D., Jewell D. P. Immunoglobulin production by isolated intestinal mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1989 Oct;78(1):37–41. [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


