Abstract
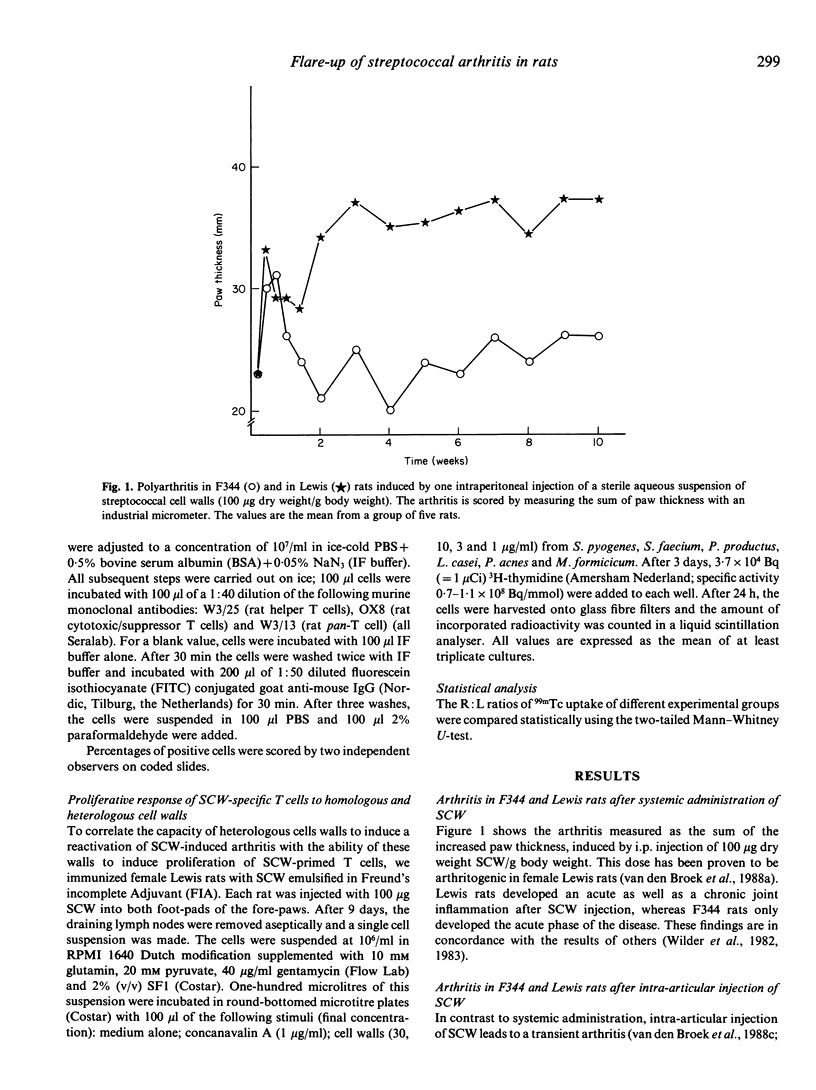
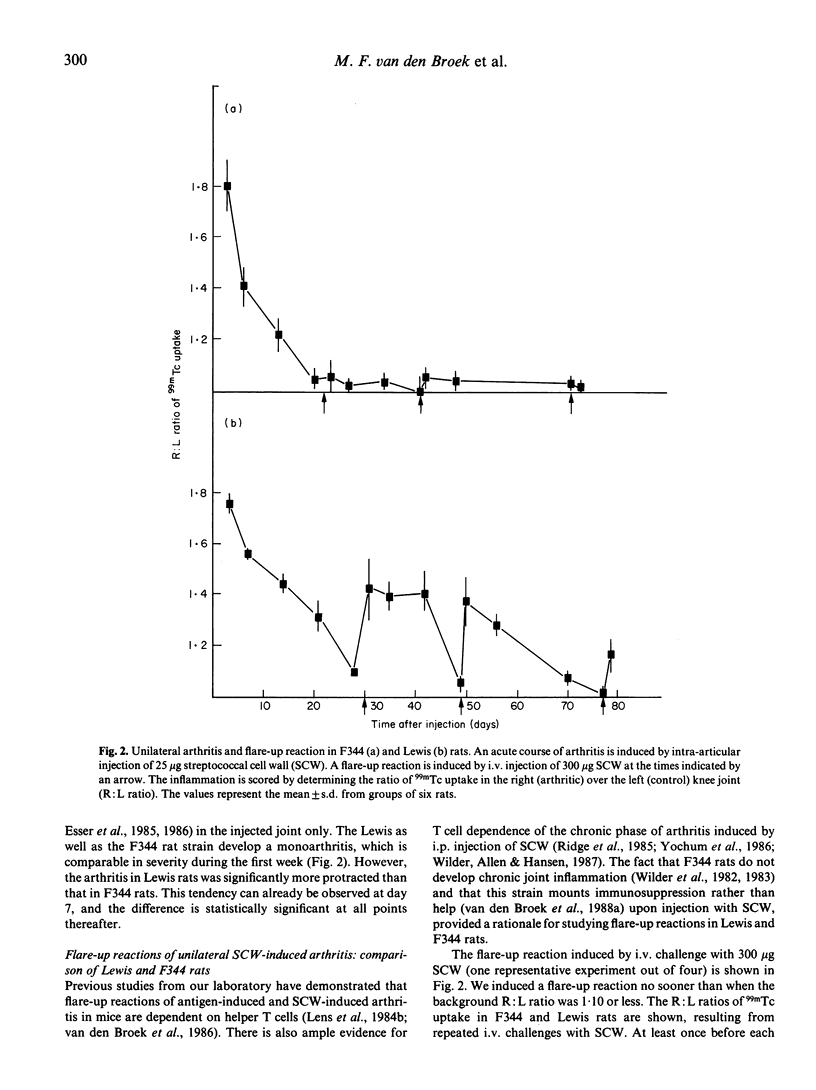
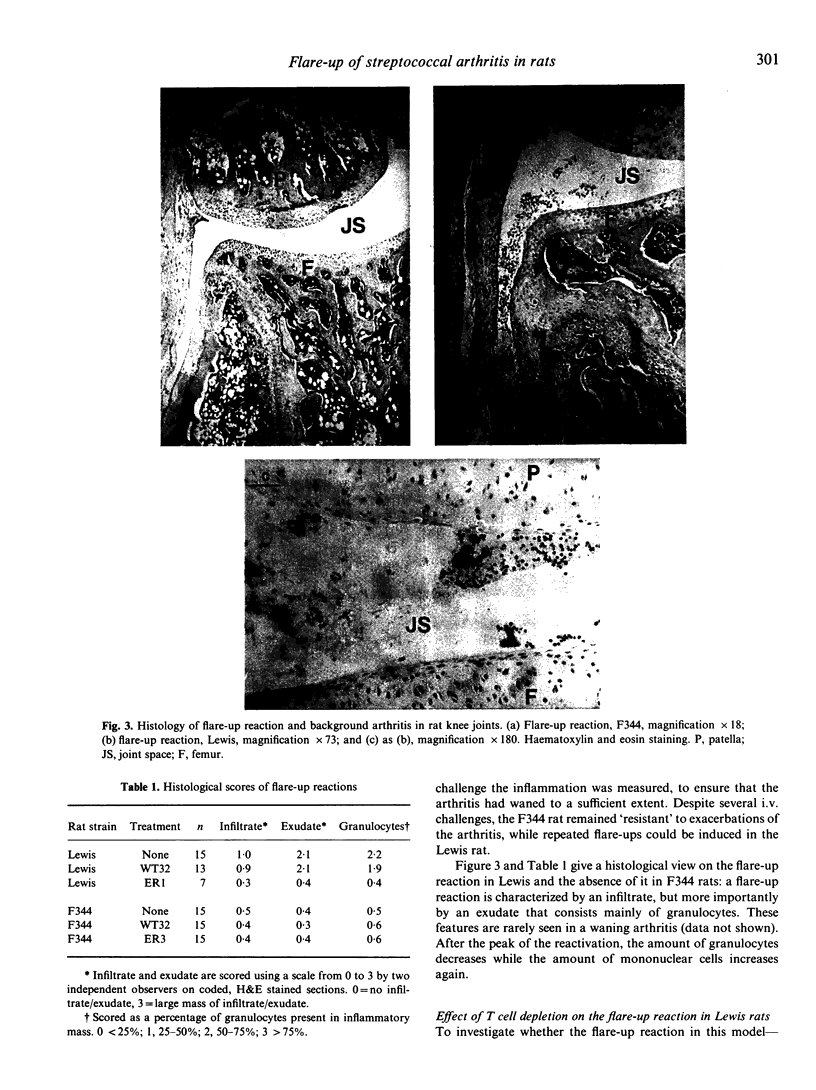
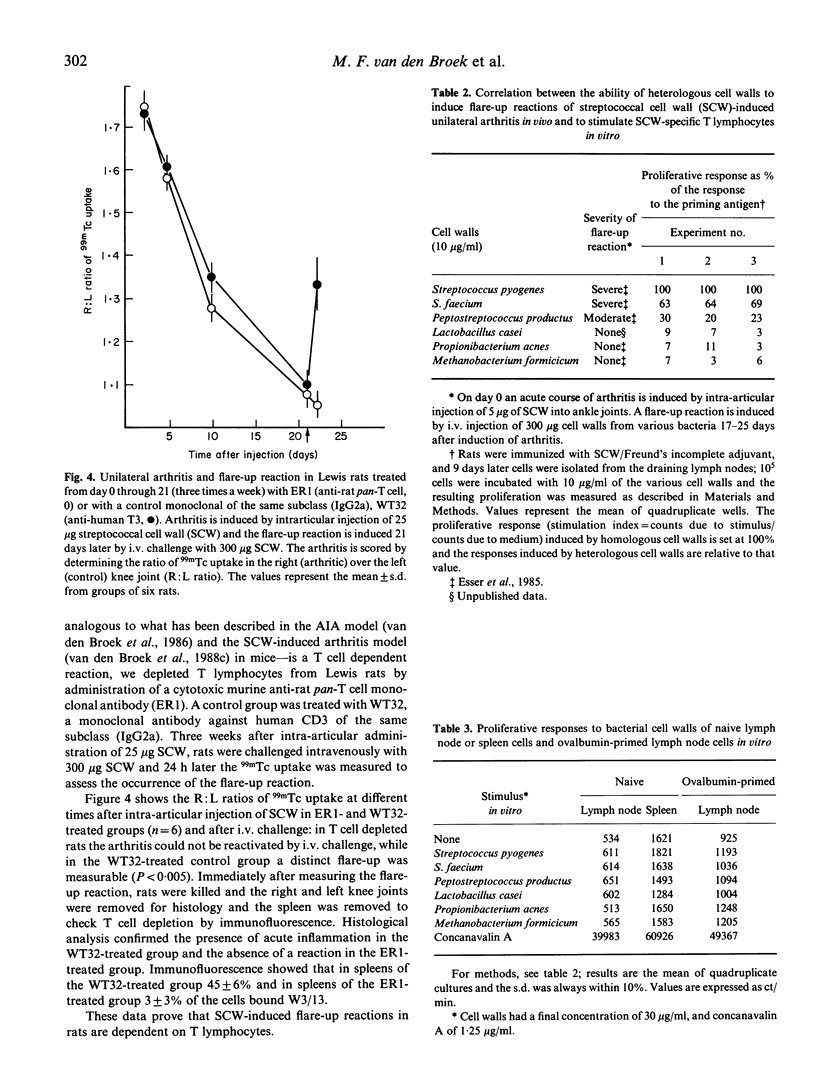
One i.p. injection of a sterile suspension of streptococcal cell walls (SCW) induces chronic erosive polyarthritis in susceptible Lewis rats, but not in resistant F344 or nude Lewis rats. Because continuous exacerbations may be one possible mechanism underlying chronic disease, we studied the mechanism of these flare-up reactions in Lewis and F344 rats. Injection of SCW into the right knee joint of rats induced a transient monoarthritis in both strains. Reactivation of the subsided arthritis by i.v. administration of the same antigen could be evoked only in the Lewis rat. Even repeated i.v. challenges with SCW failed to induce a flare-up reaction in the F344 rat, while the Lewis rat went through an exacerbation after every challenge. Removal of T lymphocytes by monoclonal antibodies before induction of an exacerbation rendered Lewis rats refractory to flare-up reactions, thus indicating the T cell-dependence of this reaction. Furthermore, when cell walls from heterologous bacteria were tested for their capacity to induce exacerbations of SCW-induced monoarthritis and to induce proliferation of SCW-specific T lymphocytes in vitro, a strong correlation between both features was found, again pointing to a role for SCW-specific T cells in exacerbations. Together, these data support our hypothesis that chronic arthritis is the result from repeated reactivations of a waning arthritis which are dependent on antigen-specific T lymphocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Calandra G. B., Wilder R. L. Cutaneous inflammatory reactions to group A streptococcal cell wall fragments in Fisher and Lewis inbred rats. Infect Immun. 1983 Nov;42(2):796–801. doi: 10.1128/iai.42.2.796-801.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. B., Malone D. G., Wahl S. M., Calandra G. B., Wilder R. L. Role of the thymus in streptococcal cell wall-induced arthritis and hepatic granuloma formation. Comparative studies of pathology and cell wall distribution in athymic and euthymic rats. J Clin Invest. 1985 Sep;76(3):1042–1056. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderle S. K., Allen J. B., Wilder R. L., Eisenberg R. A., Cromartie W. J., Schwab J. H. Measurement of streptococcal cell wall in tissues of rats resistant or susceptible to cell wall-induced chronic erosive arthritis. Infect Immun. 1985 Sep;49(3):836–837. doi: 10.1128/iai.49.3.836-837.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackertz D., Mitchell G. F., Mackay I. R. Antigen-induced arthritis in mice. I. Induction of arthritis in various strains of mice. Arthritis Rheum. 1977 Apr;20(3):841–850. doi: 10.1002/art.1780200314. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Kuwabara T., Nussenblatt R. B. Characterization of a suppressor cell line which downgrades experimental autoimmune uveoretinitis in the rat. J Immunol. 1988 Apr 15;140(8):2579–2584. [PubMed] [Google Scholar]
- Cromartie W. J., Craddock J. G., Schwab J. H., Anderle S. K., Yang C. H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977 Dec 1;146(6):1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R., Fox A., Greenblatt J. J., Anderle S. K., Cromartie W. J., Schwab J. H. Measurement of bacterial cell wall in tissues by solid-phase radioimmunoassay: correlation of distribution and persistence with experimental arthritis in rats. Infect Immun. 1982 Oct;38(1):127–135. doi: 10.1128/iai.38.1.127-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser R. E., Anderle S. K., Chetty C., Stimpson S. A., Cromartie W. J., Schwab J. H. Comparison of inflammatory reactions induced by intraarticular injection of bacterial cell wall polymers. Am J Pathol. 1986 Feb;122(2):323–334. [PMC free article] [PubMed] [Google Scholar]
- Esser R. E., Stimpson S. A., Cromartie W. J., Schwab J. H. Reactivation of streptococcal cell wall-induced arthritis by homologous and heterologous cell wall polymers. Arthritis Rheum. 1985 Dec;28(12):1402–1411. doi: 10.1002/art.1780281213. [DOI] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Fox A., Brown R. R., Anderle S. K., Chetty C., Cromartie W. J., Gooder H., Schwab J. H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982 Mar;35(3):1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J. J., Hunter N., Schwab J. H. Antibody response to streptococcal cell wall antigens associated with experimental arthritis in rats. Clin Exp Immunol. 1980 Dec;42(3):450–457. [PMC free article] [PubMed] [Google Scholar]
- Lens J. W., van den Berg W. B., van de Putte L. B., Berden J. H., Lems S. P. Flare-up of antigen-induced arthritis in mice after challenge with intravenous antigen: effects of pre-treatment with cobra venom factor and anti-lymphocyte serum. Clin Exp Immunol. 1984 Sep;57(3):520–528. [PMC free article] [PubMed] [Google Scholar]
- Lens J. W., van den Berg W. B., van de Putte L. B. Flare-up of antigen-induced arthritis in mice after challenge with intravenous antigen: studies on the characteristics of and mechanisms involved in the reaction. Clin Exp Immunol. 1984 Feb;55(2):287–294. [PMC free article] [PubMed] [Google Scholar]
- Ridge S. C., Zabriske J. B., Oronsky A. L., Kerwar S. S. Streptococcal cell wall arthritis: studies with nude (athymic) inbred Lewis rats. Cell Immunol. 1985 Nov;96(1):231–234. doi: 10.1016/0008-8749(85)90354-5. [DOI] [PubMed] [Google Scholar]
- Schwab J. H., Allen J. B., Anderle S. K., Dalldorf F., Eisenberg R., Cromartie W. J. Relationship of complement to experimental arthritis induced in rats with streptococcal cell walls. Immunology. 1982 May;46(1):83–88. [PMC free article] [PubMed] [Google Scholar]
- Sedgwick J. D. Long-term depletion of CD8+ T cells in vivo in the rat: no observed role for CD8+ (cytotoxic/suppressor) cells in the immunoregulation of experimental allergic encephalomyelitis. Eur J Immunol. 1988 Apr;18(4):495–502. doi: 10.1002/eji.1830180402. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Brown R. R., Anderle S. K., Klapper D. G., Clark R. L., Cromartie W. J., Schwab J. H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986 Jan;51(1):240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Esser R. E., Carter P. B., Sartor R. B., Cromartie W. J., Schwab J. H. Lipopolysaccharide induces recurrence of arthritis in rat joints previously injured by peptidoglycan-polysaccharide. J Exp Med. 1987 Jun 1;165(6):1688–1702. doi: 10.1084/jem.165.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson S. A., Esser R. E., Cromartie W. J., Schwab J. H. Comparison of in vivo degradation of 125I-labeled peptidoglycan-polysaccharide fragments from group A and group D streptococci. Infect Immun. 1986 May;52(2):390–396. doi: 10.1128/iai.52.2.390-396.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D., Qin Y., Chluba J., Epplen J. T., Wekerle H. Suppression of experimentally induced autoimmune encephalomyelitis by cytolytic T-T cell interactions. Nature. 1988 Apr 28;332(6167):843–845. doi: 10.1038/332843a0. [DOI] [PubMed] [Google Scholar]
- Wilder R. L., Allen J. B., Hansen C. Thymus-dependent and -independent regulation of Ia antigen expression in situ by cells in the synovium of rats with streptococcal cell wall-induced arthritis. Differences in site and intensity of expression in euthymic, athymic, and cyclosporin A-treated LEW and F344 rats. J Clin Invest. 1987 Apr;79(4):1160–1171. doi: 10.1172/JCI112933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder R. L., Allen J. B., Wahl L. M., Calandra G. B., Wahl S. M. The pathogenesis of group A streptococcal cell wall-induced polyarthritis in the rat. Comparative studies in arthritis resistant and susceptible inbred rat strains. Arthritis Rheum. 1983 Dec;26(12):1442–1451. doi: 10.1002/art.1780261205. [DOI] [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]
- Yocum D. E., Allen J. B., Wahl S. M., Calandra G. B., Wilder R. L. Inhibition by cyclosporin A of streptococcal cell wall-induced arthritis and hepatic granulomas in rats. Arthritis Rheum. 1986 Feb;29(2):262–273. doi: 10.1002/art.1780290215. [DOI] [PubMed] [Google Scholar]
- de Heer E., Daha M. R., Burgers J., van Es L. A. Reestablishment of self tolerance by suppressor T-cells after active Heymann's nephritis. Cell Immunol. 1986 Mar;98(1):28–33. doi: 10.1016/0008-8749(86)90264-9. [DOI] [PubMed] [Google Scholar]
- van Eden W., Holoshitz J., Nevo Z., Frenkel A., Klajman A., Cohen I. R. Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5117–5120. doi: 10.1073/pnas.82.15.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lent P. L., van den Berg W. B., Schalkwijk J., van de Putte L. B., van den Bersselaar L. Allergic arthritis induced by cationic antigens: relationship of chronicity with antigen retention and T-cell reactivity. Immunology. 1987 Oct;62(2):265–272. [PMC free article] [PubMed] [Google Scholar]
- van den Berg W. B., van Beusekom H. J., van de Putte L. B., Zwarts W. A., van der Sluis M. Antigen handling in antigen-induced arthritis in mice: an autoradiographic and immunofluorescence study using whole joint sections. Am J Pathol. 1982 Jul;108(1):9–16. [PMC free article] [PubMed] [Google Scholar]
- van den Berg W. B., van de Putte L. B., Zwarts W. A., Joosten L. A. Electrical charge of the antigen determines intraarticular antigen handling and chronicity of arthritis in mice. J Clin Invest. 1984 Nov;74(5):1850–1859. doi: 10.1172/JCI111604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Broek M. F., van Bruggen M. C., van de Putte L. B., van den Berg W. B. T cell responses to streptococcal antigens in rats: relation to susceptibility to streptococcal cell wall-induced arthritis. Cell Immunol. 1988 Oct 1;116(1):216–229. doi: 10.1016/0008-8749(88)90222-5. [DOI] [PubMed] [Google Scholar]
- van den Broek M. F., van den Berg W. B., van de Putte L. B. Monoclonal anti-Ia antibodies suppress the flare up reaction of antigen induced arthritis in mice. Clin Exp Immunol. 1986 Nov;66(2):320–330. [PMC free article] [PubMed] [Google Scholar]



