Abstract
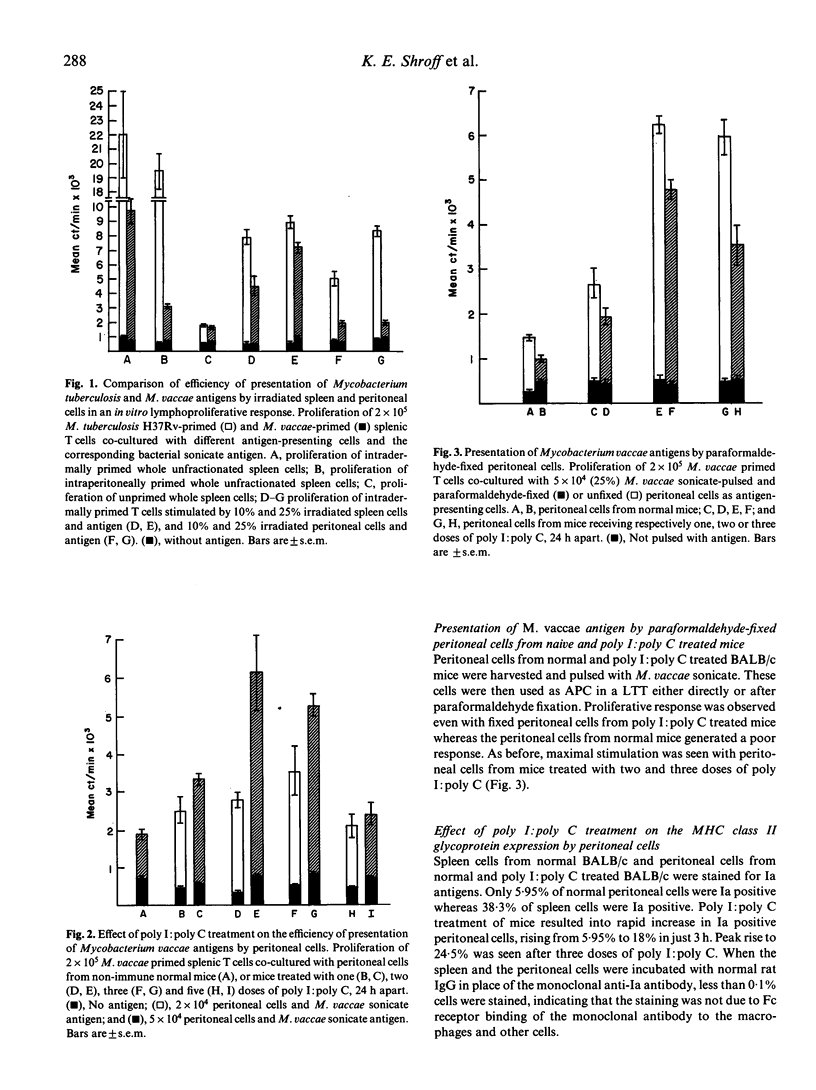
The route of immunization was observed to play a significant role in deciding the outcome of immunization with killed mycobacterial vaccines. Whereas the slow growers were immunogenic by both intraperitoneal and intradermal routes, the rapid growers were immunogenic only by intradermal route. The non-responder state of mice to Mycobacterium vaccae by i.p. route of immunization could be corrected by prior treatment with poly I:poly C, an interferon inducer, or indomethacin, a prostaglandin inhibitor. Antigen-presenting efficiency of peritoneal and spleen cells were compared employing M. vaccae and M. tuberculosis H37Rv primed T cells and corresponding sonicates as antigens in an in vitro lymphocyte transformation test. Irradiated spleen cells presented both the antigens efficiently. However, with peritoneal cells as antigen-presenting cells, proliferative response against only M. tuberculosis was observed; proliferation of M. vaccae primed T cells was very poor. Peritoneal cells of poly I:poly C treated mice showed distinct improvement in their efficiency of presentation; even paraformaldehyde-fixed peritoneal cells gave an efficient stimulation with M. vaccae. The percentage of Ia-positive fraction in peritoneal cells was very low (5.95%) in comparison with spleen cells (38.37%). Poly I:poly C treatment resulted in increase in the Ia-positive cell fraction of the peritoneal cells to 24.5%.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Beller D. I., Kiely J. M., Unanue E. R. Regulation of macrophage populations. I. Preferential induction of Ia-rich peritoneal exudates by immunologic stimuli. J Immunol. 1980 Mar;124(3):1426–1432. [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- DOUB L., YOUMANS G. P. Studies in tuberculosis chemotherapy; a simple primary aromatic amines, in vitro and in vivo. Am Rev Tuberc. 1950 Mar;61(3):407–421. [PubMed] [Google Scholar]
- GRAY D. F., JENNINGS P. A. Allergy in experimental mouse tuberculosis. Am Rev Tuberc. 1955 Aug;72(2):171–195. doi: 10.1164/artpd.1955.72.2.171. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Katz D. R. Differences in accessory cell functions. Immunobiology. 1984 Dec;168(3-5):134–140. doi: 10.1016/S0171-2985(84)80104-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lenzini L., Rottoli P., Rottoli L. The spectrum of human tuberculosis. Clin Exp Immunol. 1977 Feb;27(2):230–237. [PMC free article] [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Convit J., Rubinstein A., Bloom B. R. Activated suppressor T cells in leprosy. J Immunol. 1982 Nov;129(5):1946–1951. [PubMed] [Google Scholar]
- Ottenhoff T. H., de Vries R. R. HLA class II immune response and suppression genes in leprosy. Int J Lepr Other Mycobact Dis. 1987 Sep;55(3):521–534. [PubMed] [Google Scholar]
- Poulter L. W., Pandolph C. R. Mechanisms of immunity to leishmaniasis. IV. Significance of lymphatic drainage from the site of infection. Clin Exp Immunol. 1982 May;48(2):396–402. [PMC free article] [PubMed] [Google Scholar]
- Shepard C. C., Walker L. L., Van Landingham R. M., Ye S. Z. Sensitization or tolerance to Mycobacterium leprae antigen by route of injection. Infect Immun. 1982 Nov;38(2):673–680. doi: 10.1128/iai.38.2.673-680.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Sreevatsa, Gupta S. K., Sengupta U. Comparative study of immunizing and delayed hypersensitivity eliciting antigens of Mycobacterium leprae, M. tuberculosis, M. vaccae, and M. bovis (BCG). Int J Lepr Other Mycobact Dis. 1987 Mar;55(1):42–53. [PubMed] [Google Scholar]
- Stanford J. L., Rook G. A., Convit J., Godal T., Kronvall G., Rees R. J., Walsh G. P. Preliminary taxonomic studies on the leprosy bacillus. Br J Exp Pathol. 1975 Dec;56(6):579–585. [PMC free article] [PubMed] [Google Scholar]
- Watson S. R., Morrison N. E., Collins F. M. Delayed hypersensitivity responses in mice and guinea pigs to Mycobacterium leprae, Mycobacterium vaccae, and Mycobacterium nonchromogenicum cytoplasmic proteins. Infect Immun. 1979 Jul;25(1):229–236. doi: 10.1128/iai.25.1.229-236.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Elferink B. G., de Vries R. R., Leiker D. L., van Rood J. J. Low T lymphocyte responsiveness to Mycobacterium leprae antigens in association with HLA-DR3. Clin Exp Immunol. 1984 Jan;55(1):140–148. [PMC free article] [PubMed] [Google Scholar]
- van Eden W., de Vries R. R. Occasional review--HLA and leprosy: a re-evaluation. Lepr Rev. 1984 Jun;55(2):89–104. doi: 10.5935/0305-7518.19840012. [DOI] [PubMed] [Google Scholar]


