Abstract
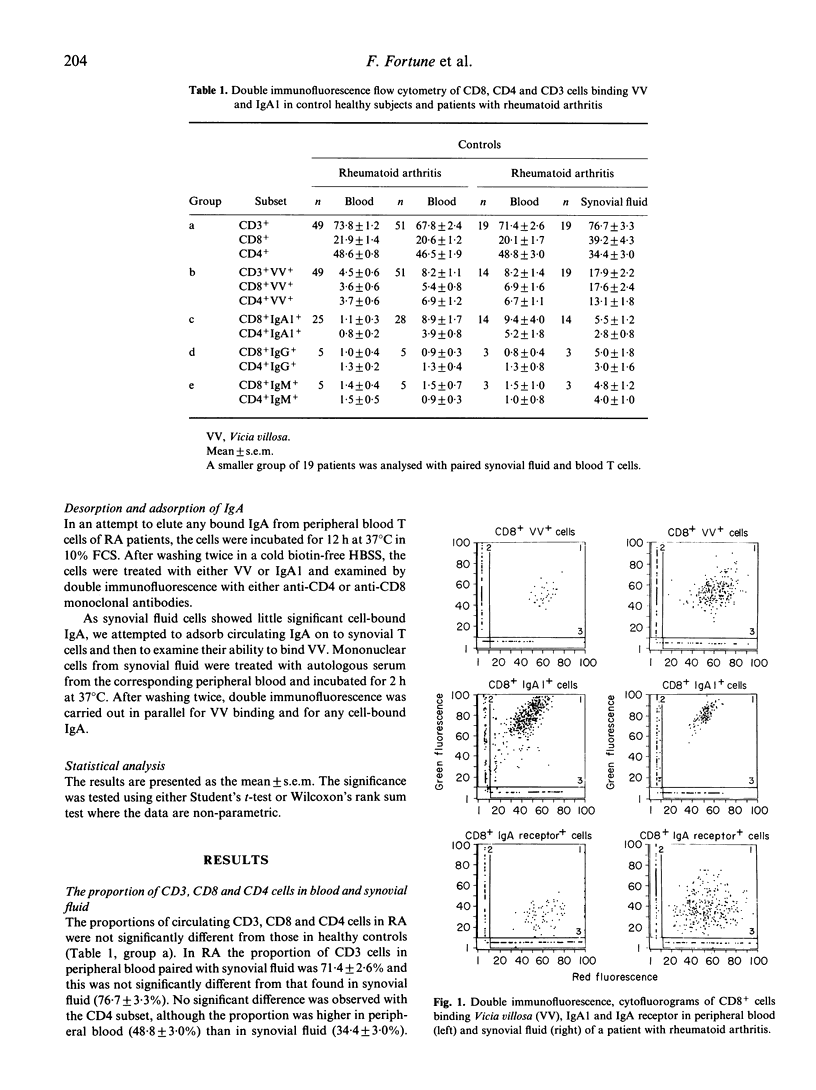
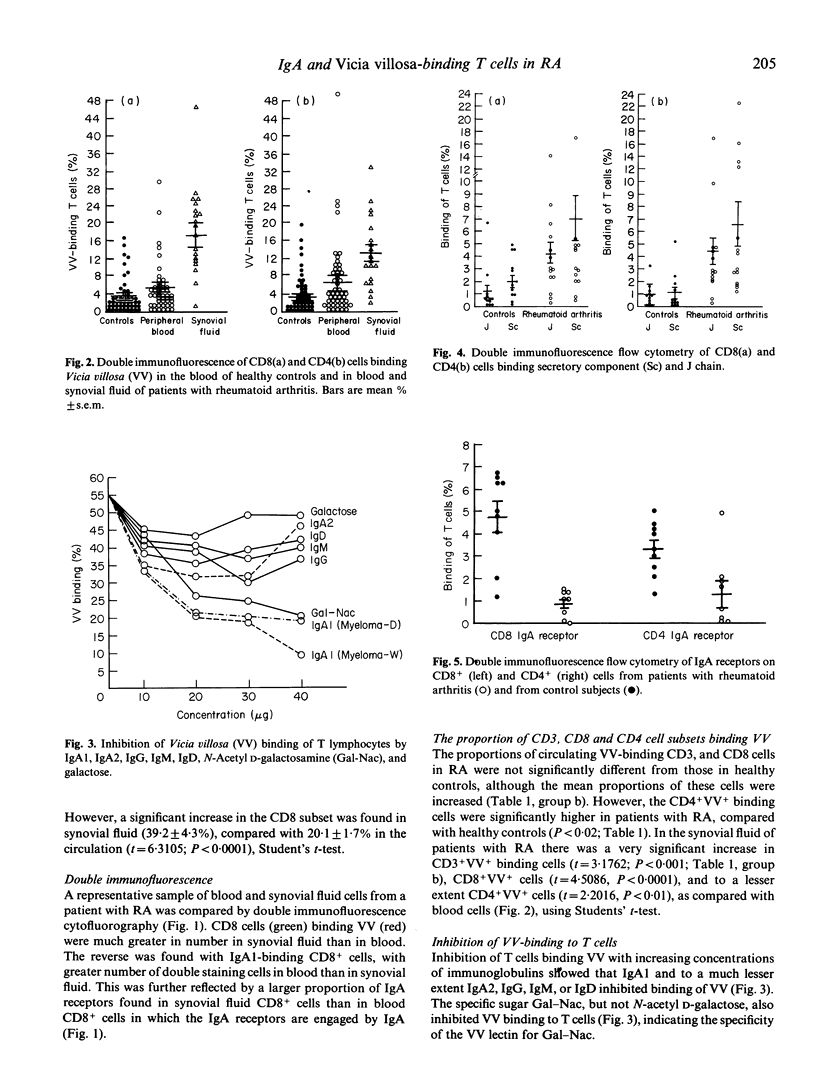
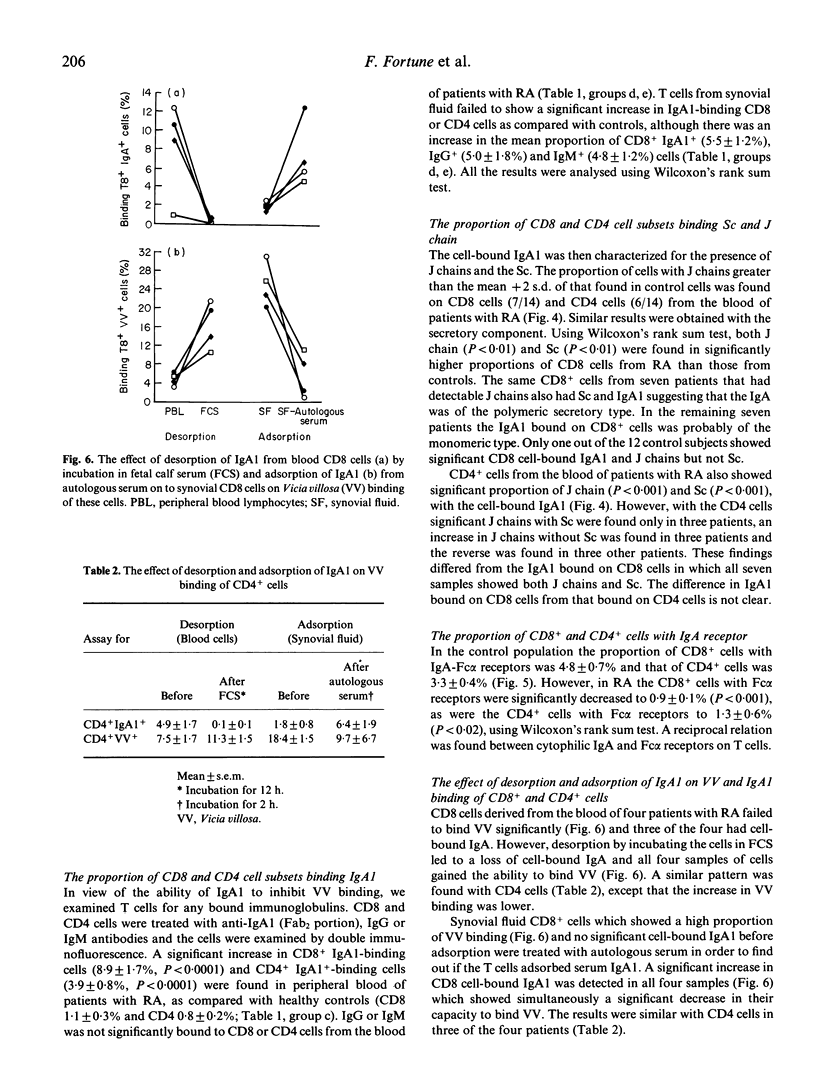
Autoimmunity may be due to augmentation of immune responses by human CD8 cells which bind the lectin Vicia villosa (VV). We have investigated T cells in rheumatoid arthritis (RA) by double immunofluorescence flow cytometry, in order to assess VV-binding CD8 and CD4 cells from the peripheral blood and synovial fluid. A significant increase in CD8+VV adherent (P less than 0.0001) and CD4+VV adherent cells (P less than 0.001) was found in synovial fluid, as compared with peripheral blood from patients with RA. A significant increase in VV-binding CD8+ or CD4+ cells was, however, not found in the blood of patients with RA, as compared with controls. We suggest that the lack of VV-binding T cells separated from blood, in contrast to those from synovial fluid, may be due to an inhibiting agent expressing N-acetyl D-galactosamine. Indeed, IgA1 is rich in N-acetyl D-galactosamine, it inhibits VV binding to T cells and is significantly bound to CD8 cells (P less than 0.001). The IgA1 was then characterized and in about half the patients J chains and secretory component was found, suggesting that the IgA1 is of the polymeric and secretory variety. IgA bound to the T cells engaged the Fc alpha receptors and a significant decrease in the Fc alpha receptors was found in CD8 cells (P less than 0.0001) and CD4 cells (P less than 0.01). Desorption studies were then carried out on CD8 and CD4 cells which showed that a loss of cell-bound IgA1 was associated with an increase in VV binding. Conversely, adsorption of IgA to T cells was associated with a loss in VV binding. The results suggest that the failure of VV binding to CD8+ and CD4+ cells from peripheral blood of patients with RA can be ascribed to cell-bound IgA1. Cytophilic IgA1 may inhibit the function of CD8+VV binding cells, thereby preventing augmentation of the systemic immune response, consistent with the lack of extra-articular disease in these patients with RA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brines R., Lehner T. Characterization of human T8+ suppressor and contrasuppressor cells, separated by the lectin Vicia villosa. Immunology. 1988 Feb;63(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- Fellowes R., Fortune F., Bergmeier L. A., Lehner T. The effect of immunization with a 14-kDa streptococcal antigen on primate T cell and B cell responses. Eur J Immunol. 1988 Apr;18(4):559–564. doi: 10.1002/eji.1830180411. [DOI] [PubMed] [Google Scholar]
- Fortune F., Lehner T. Phenotypic expression of Vicia villosa binding T cell subsets, as markers of contrasuppressor cells in systemic lupus erythematosus. Clin Exp Immunol. 1988 Oct;74(1):100–104. [PMC free article] [PubMed] [Google Scholar]
- Gershon R. K., Eardley D. D., Durum S., Green D. R., Shen F. W., Yamauchi K., Cantor H., Murphy D. B. Contrasuppression. A novel immunoregulatory activity. J Exp Med. 1981 Jun 1;153(6):1533–1546. doi: 10.1084/jem.153.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- Griffiss J. M., Goroff D. K. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J Immunol. 1983 Jun;130(6):2882–2885. [PubMed] [Google Scholar]
- Hoover R. G., Dieckgraefe B. K., Lynch R. G. T cells with Fc receptors for IgA: induction of T alpha cells in vivo and in vitro by purified IgA. J Immunol. 1981 Oct;127(4):1560–1563. [PubMed] [Google Scholar]
- Kelly C. J., Neilson E. G. Contrasuppression in autoimmunity. Abnormal contrasuppression facilitates expression of nephritogenic effector T cells and interstitial nephritis in kdkd mice. J Exp Med. 1987 Jan 1;165(1):107–123. doi: 10.1084/jem.165.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama K., Crago S. S., Itoh K., Moro I., Mestecky J. Inhibition of natural killer cell activity by IgA. Cell Immunol. 1986 Aug;101(1):143–155. doi: 10.1016/0008-8749(86)90193-0. [DOI] [PubMed] [Google Scholar]
- Kuchroo V. K., Noma T., Minami M., Dorf M. E. Down-regulation of suppressor cell induction. Immunology. 1988 Aug;64(4):633–642. [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Brines R. Phenotypic and functional characterization of human contrasuppressor cell interactions. Immunol Res. 1988;7(1):33–44. doi: 10.1007/BF02918152. [DOI] [PubMed] [Google Scholar]
- Lehner T., Jones T. A comparative study of streptococcal antigen-binding human T8+ cells and monocytes, in relation to the HLA-DRw6 locus and the helper, suppressor and contrasuppressor functions. Immunology. 1984 Oct;53(2):215–225. [PMC free article] [PubMed] [Google Scholar]
- Lydyard P. M., Fanger M. W. Characteristics and function of Fc receptors on human lymphocytes. Immunology. 1982 Sep;47(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. A., Ferrone S., Theofilopoulos A. N. Isolation of human T and B lymphocytes by rosette formation with 2-aminoethylisothiquronium bromide (AET) -treated sheep red blood cells with monkey red blood cells. J Immunol Methods. 1976;11(3-4):273–279. doi: 10.1016/0022-1759(76)90120-4. [DOI] [PubMed] [Google Scholar]
- Ptak W., Green D. R., Flood P. Cellular interactions in the adoptive transfer of contact sensitivity: characterization of an antigen-nonspecific Vicia villosa-adherent T cell needed for adoptive transfer into naive recipients. J Immunol. 1986 Sep 15;137(6):1822–1828. [PubMed] [Google Scholar]
- Sakane T., Steinberg A. D., Green I. Studies of immune functions of patients with systemic lupus erythematosus. I. Dysfunction of suppressor T-cell activity related to impaired generation of, rather than response to, suppressor cells. Arthritis Rheum. 1978 Jul-Aug;21(6):657–664. doi: 10.1002/art.1780210608. [DOI] [PubMed] [Google Scholar]
- Tollefsen S. E., Kornfeld R. The B4 lectin from Vicia villosa seeds interacts with N-acetylgalactosamine residues alpha-linked to serine or threonine residues in cell surface glycoproteins. J Biol Chem. 1983 Apr 25;258(8):5172–5176. [PubMed] [Google Scholar]
- Van Epps D. E., Williams R. C., Jr Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med. 1976 Nov 2;144(5):1227–1242. doi: 10.1084/jem.144.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978 Dec;34(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- Ziegler J. B., Hansen P., Penny R. Intrinsic lymphocyte defect in Hodgkin's disease: analysis of the phytohemagglutinin dose-response. Clin Immunol Immunopathol. 1975 Mar;3(4):451–460. doi: 10.1016/0090-1229(75)90069-0. [DOI] [PubMed] [Google Scholar]


