Abstract
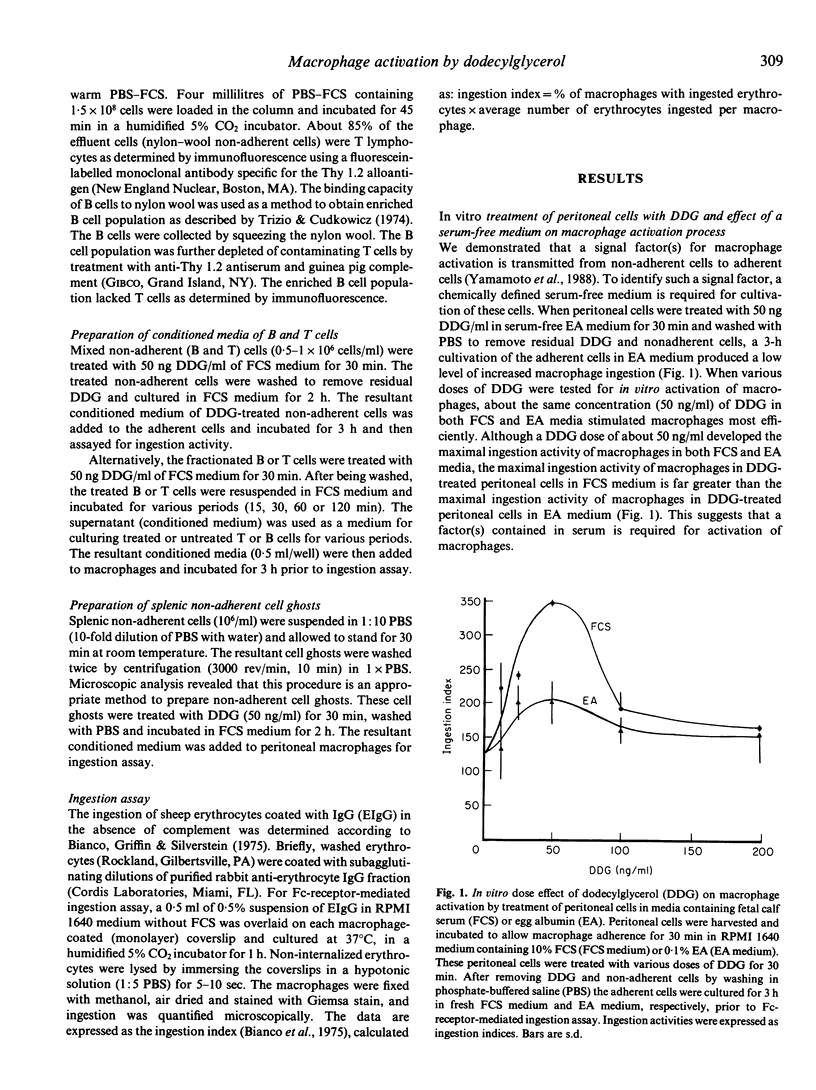
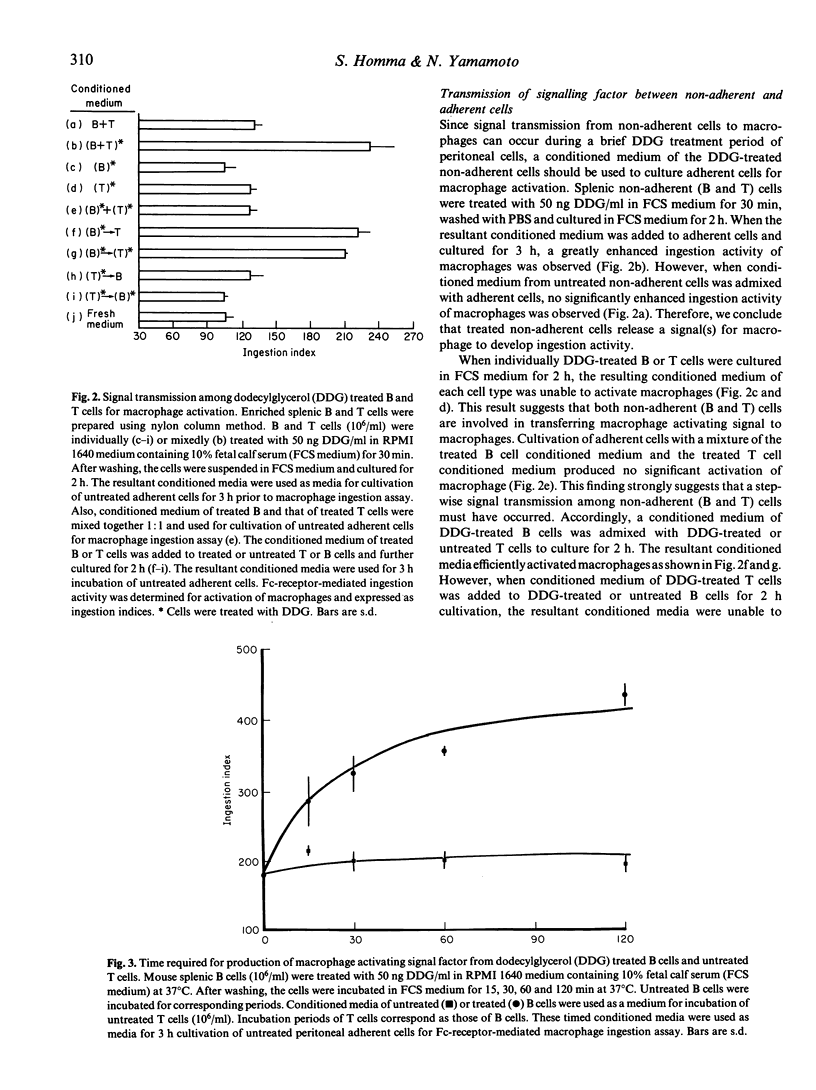
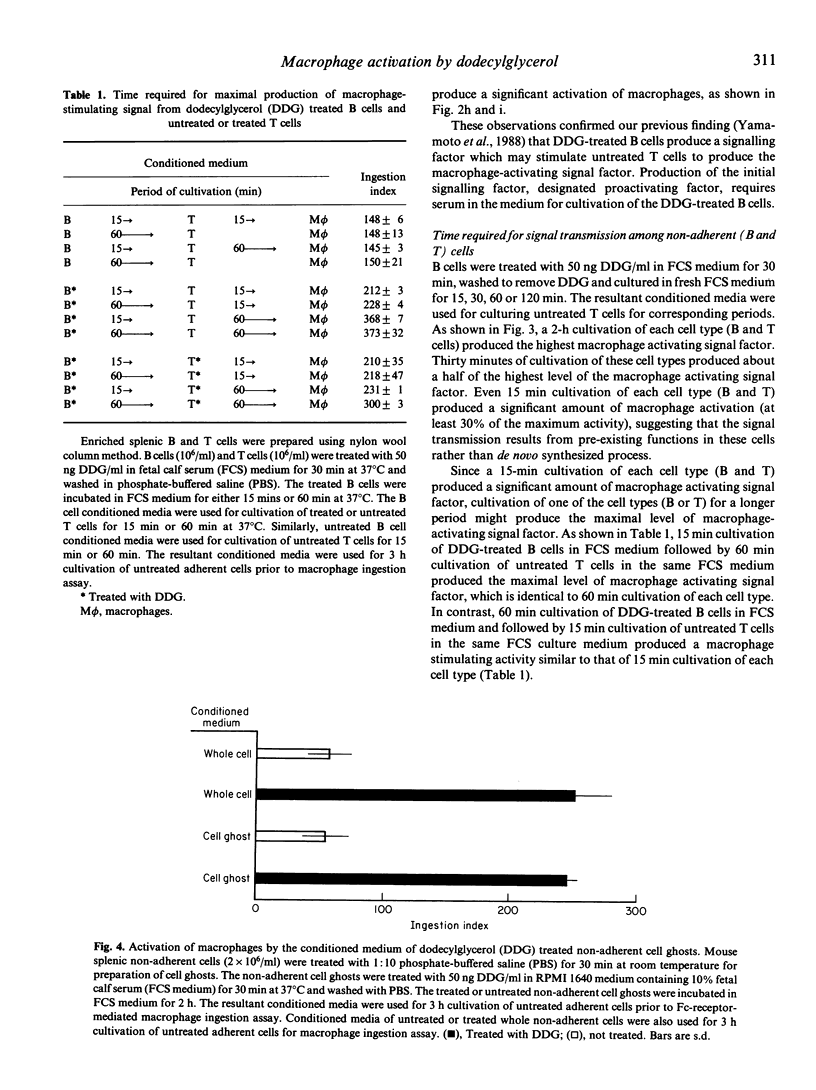
Alkylglycerols, inflammation products of cancerous membrane lipids, efficiently activate macrophages. A brief in vitro treatment (30 min) of peritoneal cells (mixture of non-adherent and adherent cells) with a small amount (50 ng/ml) of synthetic dodecylglycerol (DDG) resulted in greatly enhanced Fc-receptor-mediated ingestion activity of macrophages. However, treatment of adherent cells (macrophages) alone with DDG produced no significant enhancement of macrophage ingestion activity, implying that macrophage activation requires a contribution of non-adherent cells. DDG-treated non-adherent cells were found to generate a macrophage-activating signal factor. Studies with a serum free-0.1% egg albumin-supplemented RPMI 1640 medium revealed that a serum factor is essential for macrophage activation process. Time course analysis of stepwise transfers of conditioned media of DDG-treated or untreated B cells and T cells revealed that DDG-treated B cells rapidly transmit a factor to untreated T cells which yield the ultimate macrophage-activating factor. This signal transmission among these cells for the macrophage activation process is too rapid to allow time for synthesis of inducible gene products. Thus, we hypothesized that a serum factor is modified by the pre-existing function of DDG-treated B cells and further modified by the pre-existing function of untreated T cells to yield macrophage-activating factor. This hypothesis was confirmed by the demonstration that DDG-treated splenic non-adherent cell ghosts modify a serum factor to yield macrophage-activating factor.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BENSON B. THE DIFFERENTIATION OF MONONUCLEAR PHAGOCYTES. MORPHOLOGY, CYTOCHEMISTRY, AND BIOCHEMISTRY. J Exp Med. 1965 Jan 1;121:153–170. doi: 10.1084/jem.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constans J., Oksman F., Viau M. Binding of the apo and holo forms of the serum vitamin D-binding protein to human lymphocyte cytoplasm and membrane by indirect immunofluorescence. Immunol Lett. 1981 Aug;3(3):159–162. doi: 10.1016/0165-2478(81)90120-6. [DOI] [PubMed] [Google Scholar]
- Galbraith G. M., Galbraith R. M. Metabolic and cytoskeletal modulation of transferrin receptor mobility in mitogen-activated human lymphocytes. Clin Exp Immunol. 1980 Nov;42(2):285–293. [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Silverstein S. C. Segmental response of the macrophage plasma membrane to a phagocytic stimulus. J Exp Med. 1974 Feb 1;139(2):323–336. doi: 10.1084/jem.139.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. V., Morris H. P., Bailey J. M. Ether-lipids, -glycerol phosphate dehydrogenase, and growth rate in tumors and cultured cells. Cancer Res. 1972 Jul;32(7):1533–1538. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Machii T., Kimura H., Ueda E., Chujo T., Morita T., Katagiri S., Tagawa S., Kitani T. Distribution of Gc protein (vitamin D binding protein) on the surfaces of normal human lymphocytes and leukemic lymphocytes. Acta Haematol. 1986;75(1):26–29. doi: 10.1159/000206075. [DOI] [PubMed] [Google Scholar]
- Nel A. E., Navailles M., Emerson D. L., Goldschmidt-Clermont P., Pathak S. K., Tsang K. Y., Galbraith R. M. Altered configuration of Gc on the plasma membrane of transformed and malignant human B lymphocytes. Clin Immunol Immunopathol. 1985 Nov;37(2):191–202. doi: 10.1016/0090-1229(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Ngwenya B. Z., Yamamoto N. Activation of peritoneal macrophages by lysophosphatidylcholine. Biochim Biophys Acta. 1985 Mar 29;839(1):9–15. doi: 10.1016/0304-4165(85)90175-8. [DOI] [PubMed] [Google Scholar]
- Ngwenya B. Z., Yamamoto N. Effects of inflammation products on immune systems. Lysophosphatidylcholine stimulates macrophages. Cancer Immunol Immunother. 1986;21(3):174–182. doi: 10.1007/BF00199358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor. Sci Am. 1988 May;258(5):59-60, 69-75. doi: 10.1038/scientificamerican0588-59. [DOI] [PubMed] [Google Scholar]
- Petrini M., Emerson D. L., Galbraith R. M. Linkage between surface immunoglobulin and cytoskeleton of B lymphocytes may involve Gc protein. Nature. 1983 Nov 3;306(5938):73–74. doi: 10.1038/306073a0. [DOI] [PubMed] [Google Scholar]
- Petrini M., Galbraith R. M., Werner P. A., Emerson D. L., Arnaud P. Gc (vitamin D binding protein) binds to cytoplasm of all human lymphocytes and is expressed on B-cell membranes. Clin Immunol Immunopathol. 1984 May;31(2):282–295. doi: 10.1016/0090-1229(84)90248-4. [DOI] [PubMed] [Google Scholar]
- Snyder F., Wood R. Alkyl and alk-1-enyl ethers of glycerol in lipids from normal and neoplastic human tissues. Cancer Res. 1969 Jan;29(1):251–257. [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]
- Tyan M. L., Ness D. B. Mouse blood leukocytes: in vitro primary and secondary responses to two synthetic polypeptides. J Immunol. 1971 Jan;106(1):289–291. [PubMed] [Google Scholar]
- Weber N. Metabolism of orally administered rac-1-O-[1'-14C]dodecylglycerol and nutritional effects of dietary rac-1-O-dodecylglycerol in mice. J Lipid Res. 1985 Dec;26(12):1412–1420. [PubMed] [Google Scholar]
- Yamamoto N., Ngwenya B. Z. Activation of mouse peritoneal macrophages by lysophospholipids and ether derivatives of neutral lipids and phospholipids. Cancer Res. 1987 Apr 15;47(8):2008–2013. [PubMed] [Google Scholar]
- Yamamoto N., Ngwenya B. Z., Sery T. W., Pieringer R. A. Activation of macrophages by ether analogues of lysophospholipids. Cancer Immunol Immunother. 1987;25(3):185–192. doi: 10.1007/BF00199146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., St Claire D. A., Jr, Homma S., Ngwenya B. Z. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res. 1988 Nov 1;48(21):6044–6049. [PubMed] [Google Scholar]
- Zbar B., Tanaka T. Immunotherapy of cancer: regression of tumors after intralesional injection of living Mycobacterium bovis. Science. 1971 Apr 16;172(3980):271–273. doi: 10.1126/science.172.3980.271. [DOI] [PubMed] [Google Scholar]


