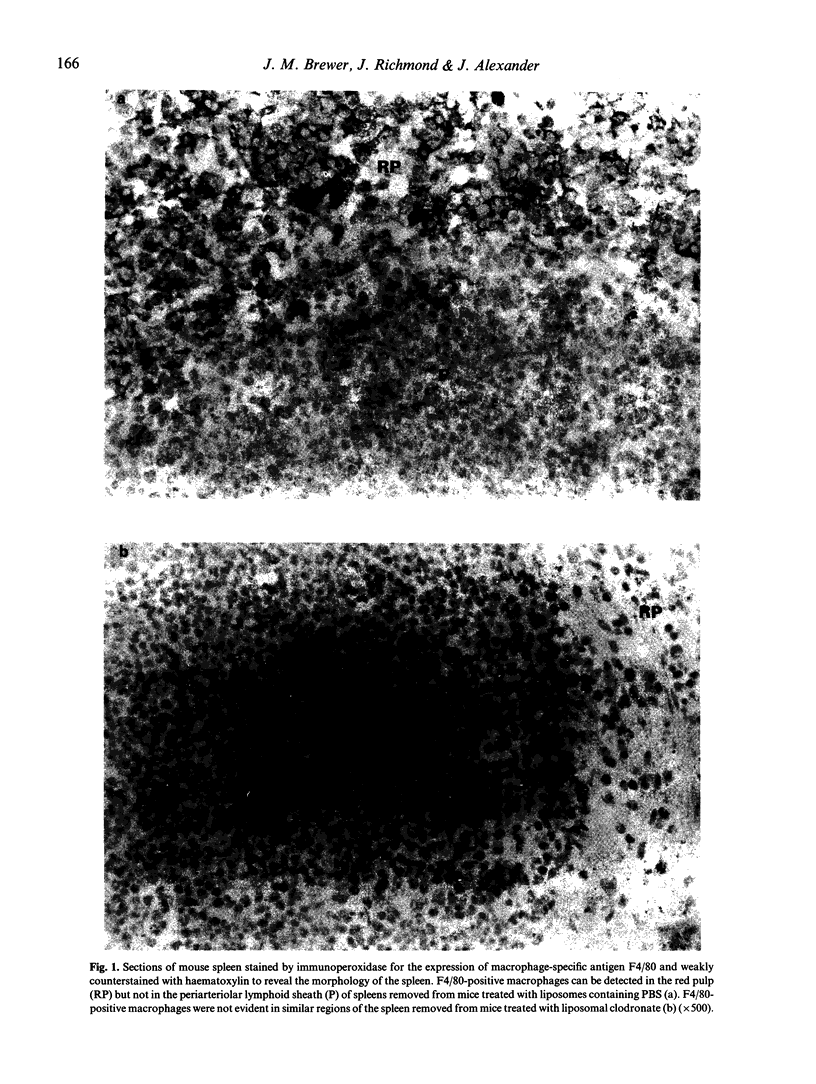
Abstract
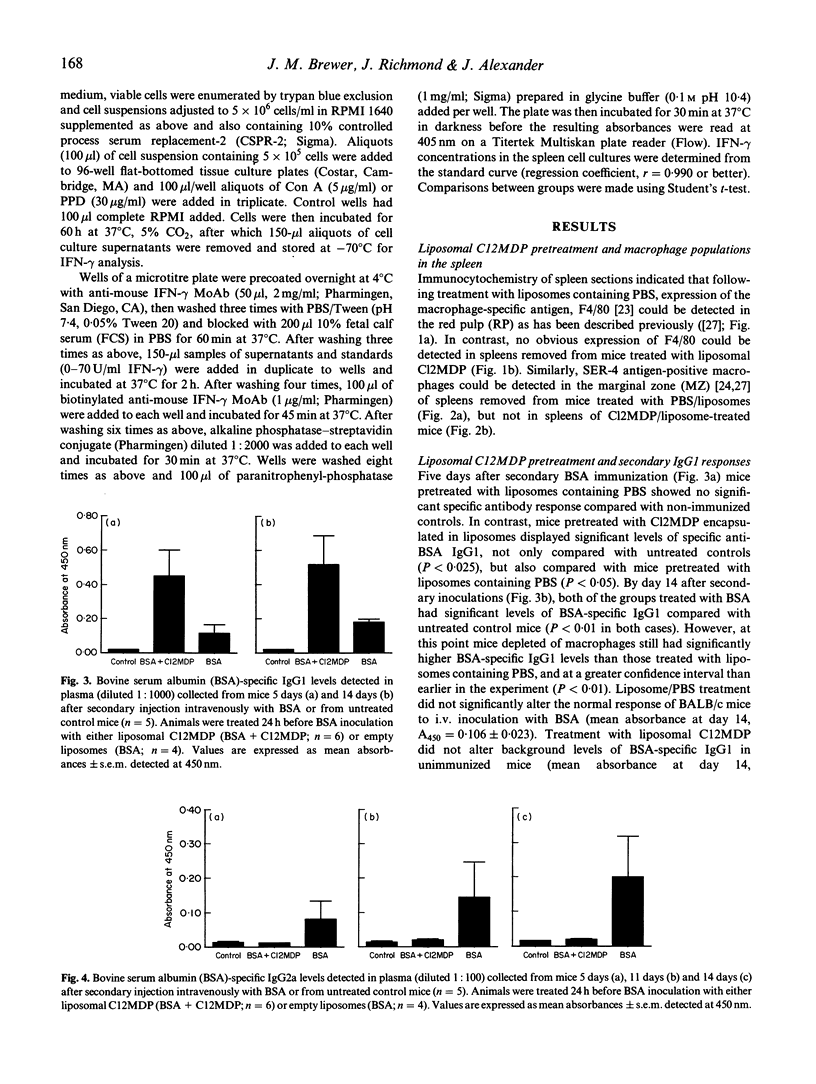
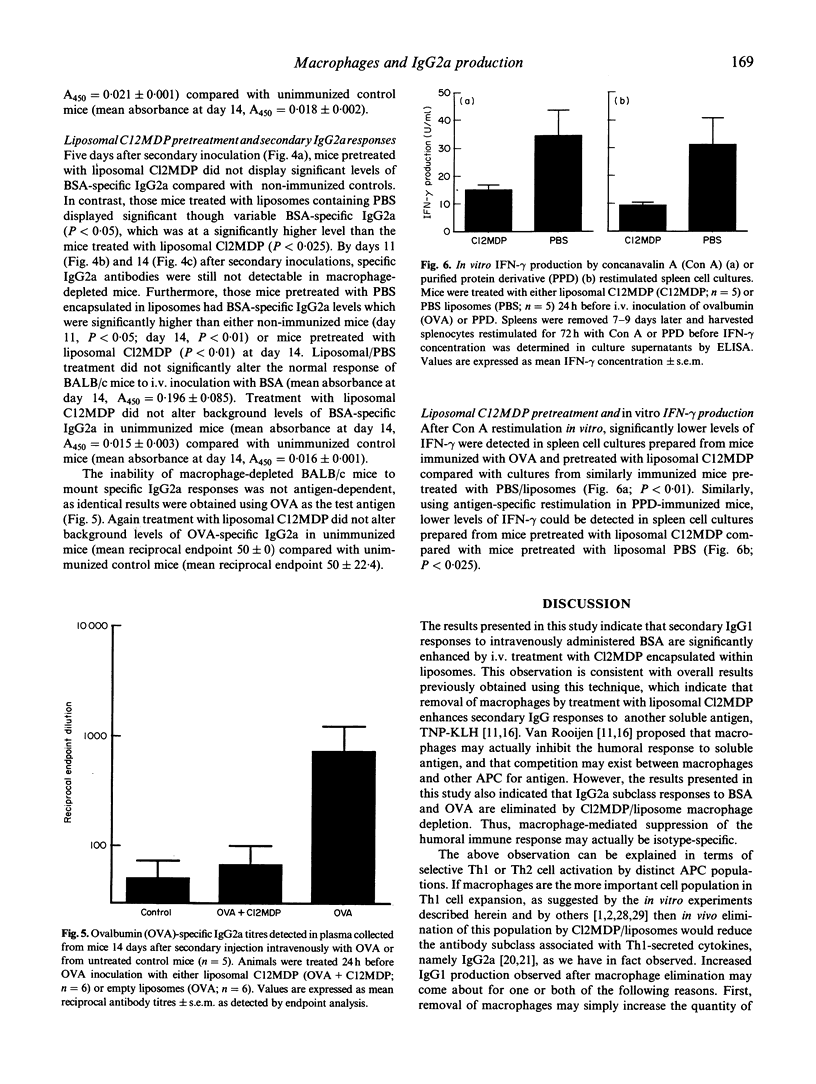
BALB/c mice were depleted of macrophages by intravenous inoculation of dichloromethylene diphosphonate entrapped in liposomes 24 h before primary and 24 h before secondary sensitization intravenously with 100 micrograms bovine serum albumin (BSA) or ovalbumin (OVA). The effectiveness of macrophage depletion was confirmed by immunocytochemistry. Five days and 14 days after secondary challenge with BSA, plasma samples from these and control mice inoculated with empty liposomes were examined for the production of BSA-specific IgG1 and IgG2a antibodies. Macrophage depletion resulted in a significantly increased production of the Th2 lymphocyte-associated IgG1 isotype, while the production of specific IgG2a antibodies, produced under the influence of Th1 cells, was totally ablated. Similar results were obtained when OVA was used as the test antigen. Furthermore, analysis of interferon-gamma (IFN-gamma) production after antigen or concanavalin A (Con A) restimulation in vitro indicated that macrophage depletion in vivo significantly reduced production of this Th1 cell-associated cytokine. These results provide strong in vivo and in vitro evidence for the macrophage being the antigen-presenting cell population responsible for Th1 cell activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbas A. K., Williams M. E., Burstein H. J., Chang T. L., Bossu P., Lichtman A. H. Activation and functions of CD4+ T-cell subsets. Immunol Rev. 1991 Oct;123:5–22. doi: 10.1111/j.1600-065x.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Bogen S. A., Fogelman I., Abbas A. K. Analysis of IL-2, IL-4, and IFN-gamma-producing cells in situ during immune responses to protein antigens. J Immunol. 1993 May 15;150(10):4197–4205. [PubMed] [Google Scholar]
- Bogen S. A., Weinberg D. S., Abbas A. K. Histologic analysis of T lymphocyte activation in reactive lymph nodes. J Immunol. 1991 Sep 1;147(5):1537–1541. [PubMed] [Google Scholar]
- Boog C. J., Boes J., Melief C. J. Role of dendritic cells in the regulation of class I restricted cytotoxic T lymphocyte responses. J Immunol. 1988 May 15;140(10):3331–3337. [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Brewer J. M., Alexander J. The adjuvant activity of non-ionic surfactant vesicles (niosomes) on the BALB/c humoral response to bovine serum albumin. Immunology. 1992 Apr;75(4):570–575. [PMC free article] [PubMed] [Google Scholar]
- Chang T. L., Shea C. M., Urioste S., Thompson R. C., Boom W. H., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. III. Responses of IL-2- and IL-4-producing (Th1 and Th2) clones to antigens presented by different accessory cells. J Immunol. 1990 Nov 1;145(9):2803–2808. [PubMed] [Google Scholar]
- Claassen E., Kors N., van Rooijen N. Immunomodulation with liposomes: the immune response elicited by liposomes with entrapped dichloromethylene-diphosphonate and surface-associated antigen or hapten. Immunology. 1987 Apr;60(4):509–515. [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989 Apr 1;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrick J. E., Campbell P. A., Staerz U. D. Macrophages as accessory cells for class I MHC-restricted immune responses. J Immunol. 1991 Nov 1;147(9):2846–2851. [PubMed] [Google Scholar]
- Finkelman F. D., Holmes J., Katona I. M., Urban J. F., Jr, Beckmann M. P., Park L. S., Schooley K. A., Coffman R. L., Mosmann T. R., Paul W. E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. Alloreactive murine CD8+ T cell clones secrete the Th1 pattern of cytokines. J Immunol. 1990 Mar 1;144(5):1744–1752. [PubMed] [Google Scholar]
- Gajewski T. F., Fitch F. W. Anti-proliferative effect of IFN-gamma in immune regulation. I. IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988 Jun 15;140(12):4245–4252. [PubMed] [Google Scholar]
- Gajewski T. F., Joyce J., Fitch F. W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and recombinant IFN-gamma. J Immunol. 1989 Jul 1;143(1):15–22. [PubMed] [Google Scholar]
- Gajewski T. F., Pinnas M., Wong T., Fitch F. W. Murine Th1 and Th2 clones proliferate optimally in response to distinct antigen-presenting cell populations. J Immunol. 1991 Mar 15;146(6):1750–1758. [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Grun J. L., Maurer P. H. Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol. 1989 Jun;121(1):134–145. doi: 10.1016/0008-8749(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Collins D. S., Kanagawa O., Unanue E. R. Liposome-encapsulated antigens engender lysosomal processing for class II MHC presentation and cytosolic processing for class I presentation. J Immunol. 1991 Nov 1;147(9):2860–2863. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R. Murine CD4+ T cell subsets defined. J Exp Med. 1988 Nov 1;168(5):1825–1838. doi: 10.1084/jem.168.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeg K., Kuon W., Wagner H. Vaccination of class I major histocompatibility complex (MHC)-restricted murine CD8+ cytotoxic T lymphocytes towards soluble antigens: immunostimulating-ovalbumin complexes enter the class I MHC-restricted antigen pathway and allow sensitization against the immunodominant peptide. Eur J Immunol. 1991 Jun;21(6):1523–1527. doi: 10.1002/eji.1830210628. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Carding S., Jones B., Murray J., Portoles P., Rasmussen R., Rojo J., Saizawa K., West J., Bottomly K. CD4+ T cells: specificity and function. Immunol Rev. 1988 Jan;101:39–80. doi: 10.1111/j.1600-065x.1988.tb00732.x. [DOI] [PubMed] [Google Scholar]
- Kagan E., Hartmann D. P. Elimination of macrophages with silica and asbestos. Methods Enzymol. 1984;108:325–335. doi: 10.1016/s0076-6879(84)08099-x. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Liano D., HayGlass K. A., Benacerraf B., Sy M. S., Abbas A. K. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988 Jun 1;140(11):3773–3778. [PubMed] [Google Scholar]
- Lopes L. M., Chain B. M. Liposome-mediated delivery stimulates a class I-restricted cytotoxic T cell response to soluble antigen. Eur J Immunol. 1992 Jan;22(1):287–290. doi: 10.1002/eji.1830220143. [DOI] [PubMed] [Google Scholar]
- Luqman M., Greenbaum L., Lu D., Bottomly K. Differential effect of interleukin 1 on naive and memory CD4+ T cells. Eur J Immunol. 1992 Jan;22(1):95–100. doi: 10.1002/eji.1830220115. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Street N. F., Budd R., O'Garra A., Fong T. A., Bond M. W., Moore K. W., Sher A., Fiorentino D. F. Diversity of cytokine synthesis and function of mouse CD4+ T cells. Immunol Rev. 1991 Oct;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Pomeroy C., Filice G. A. Effect of intravenous silica on the course of Nocardia asteroides pneumonia. Infect Immun. 1988 Sep;56(9):2507–2511. doi: 10.1128/iai.56.9.2507-2511.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Zhou F., Nair S., Huang L., Rouse B. T. In vivo cytotoxic T lymphocyte induction with soluble proteins administered in liposomes. J Immunol. 1992 Mar 1;148(5):1585–1589. [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987 May 1;138(9):2848–2856. [PubMed] [Google Scholar]
- Sacks D. L., Scott P. A., Asofsky R., Sher F. A. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984 Apr;132(4):2072–2077. [PubMed] [Google Scholar]
- Schmitz J., Radbruch A. Distinct antigen presenting cell-derived signals induce TH cell proliferation and expression of effector cytokines. Int Immunol. 1992 Jan;4(1):43–51. doi: 10.1093/intimm/4.1.43. [DOI] [PubMed] [Google Scholar]
- Shek P. N., Lukovich S. The role of macrophages in promoting the antibody response mediated by liposome-associated protein antigens. Immunol Lett. 1982;5(6):305–309. doi: 10.1016/0165-2478(82)90118-3. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Su D., Van Rooijen N. The role of macrophages in the immunoadjuvant action of liposomes: effects of elimination of splenic macrophages on the immune response against intravenously injected liposome-associated albumin antigen. Immunology. 1989 Mar;66(3):466–470. [PMC free article] [PubMed] [Google Scholar]
- Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989 Nov 13;124(1):1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- Weaver C. T., Unanue E. R. The costimulatory function of antigen-presenting cells. Immunol Today. 1990 Feb;11(2):49–55. doi: 10.1016/0167-5699(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Witmer M. D., Steinman R. M. The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer's patch. Am J Anat. 1984 Jul;170(3):465–481. doi: 10.1002/aja.1001700318. [DOI] [PubMed] [Google Scholar]
- Zhou F., Rouse B. T., Huang L. Induction of cytotoxic T lymphocytes in vivo with protein antigen entrapped in membranous vehicles. J Immunol. 1992 Sep 1;149(5):1599–1604. [PubMed] [Google Scholar]
- van Rooijen N. Antigen processing and presentation in vivo: the microenvironment as a crucial factor. Immunol Today. 1990 Dec;11(12):436–439. doi: 10.1016/0167-5699(90)90171-5. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Kors N., Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989 Feb;45(2):97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. Liposomes as an in vivo tool to study and manipulate macrophage function. Res Immunol. 1992 Feb;143(2):177–178. doi: 10.1016/s0923-2494(92)80160-m. [DOI] [PubMed] [Google Scholar]
- van Rooijen N. Macrophages as accessory cells in the in vivo humoral immune response: from processing of particulate antigens to regulation by suppression. Semin Immunol. 1992 Aug;4(4):237–245. [PubMed] [Google Scholar]