Abstract
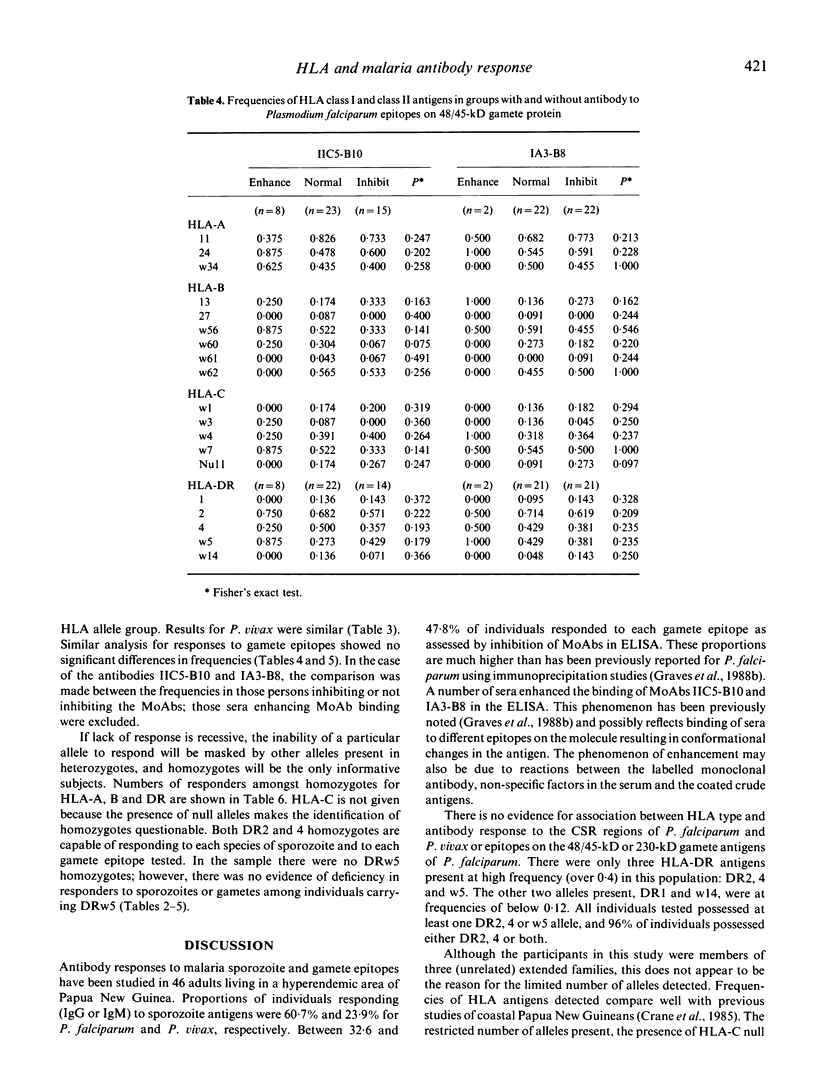
HLA-A,B,C and DR types were determined for 46 adults living in the Madang area of Papua New Guinea. Sera from these individuals were tested by ELISA for antibodies against: (i) sonicated schizont extract of Plasmodium falciparum; (ii) circumsporozoite repeat regions of P. falciparum and P. vivax; and (iii) epitopes on the 230 and 48/45 kD gametocyte antigens of P. falciparum. All sera were from highly immune individuals and reacted strongly to the schizont antigen. The proportions responding to circumsporozoite repeat regions were 60.7% and 23.9% for P. falciparum and P. vivax, respectively. Between 32.6 and 47.8% of adults responded to each gametocyte epitope as assessed by inhibition of monoclonal antibodies. The limited number of alleles present at each HLA locus which is characteristic of coastal Papua New Guinea was observed. Five HLA-DR alleles were detected, of which only three (HLA-DR2, 4 and w5) were present at frequencies over 0.12. All individuals possessed at least one DR2,4 or w5 allele, and 96% of individuals possessed DR2, or 4 or both. There was no evidence for association between HLA type and antibody response to circumsporozoite repeat regions or the gametocyte epitopes. Homozygotes for DR2 and 4 were able to respond to each antigen. These results imply that either there is no HLA restriction of the response to these antigens or that each DR type is responding to a different variant of the T-epitope. Even in the latter case the results are encouraging for the prospects of inclusion of an HLA-restricted T-epitope in a malaria vaccine for Papua New Guinea since a limited number of versions would be required to cover a population with an HLA profile similar to that in Madang.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou W. R., Hoffman S. L., Sherwood J. A., Hollingdale M. R., Neva F. A., Hockmeyer W. T., Gordon D. M., Schneider I., Wirtz R. A., Young J. F. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987 Jun 6;1(8545):1277–1281. doi: 10.1016/s0140-6736(87)90540-x. [DOI] [PubMed] [Google Scholar]
- Bhatia K., Crane G. HLA and tropical splenomegaly syndrome in the Upper Watut Valley of Papua New Guinea. Hum Immunol. 1985 Aug;13(4):235–242. doi: 10.1016/0198-8859(85)90002-3. [DOI] [PubMed] [Google Scholar]
- Bhatia K., Gorogo M., Koki G. HLA-A,B,C and DR antigens in Asaro speakers of Papua New Guinea. Hum Immunol. 1984 Apr;9(4):189–200. doi: 10.1016/0198-8859(84)90024-7. [DOI] [PubMed] [Google Scholar]
- Burkot T. R., Graves P. M., Paru R., Wirtz R. A., Heywood P. F. Human malaria transmission studies in the Anopheles punctulatus complex in Papua New Guinea: sporozoite rates, inoculation rates, and sporozoite densities. Am J Trop Med Hyg. 1988 Aug;39(2):135–144. doi: 10.4269/ajtmh.1988.39.135. [DOI] [PubMed] [Google Scholar]
- Burkot T. R., Graves P. M., Wirtz R. A., Brabin B. J., Battistutta D., Cattani J. A., Maizels R. M., Alpers M. P. Differential antibody responses to Plasmodium falciparum and P. vivax circumsporozoite proteins in a human population. J Clin Microbiol. 1989 Jun;27(6):1346–1351. doi: 10.1128/jcm.27.6.1346-1351.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter R., Graves P. M., Quakyi I. A., Good M. F. Restricted or absent immune responses in human populations to Plasmodium falciparum gamete antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1989 Jan 1;169(1):135–147. doi: 10.1084/jem.169.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattani J. A., Tulloch J. L., Vrbova H., Jolley D., Gibson F. D., Moir J. S., Heywood P. F., Alpers M. P., Stevenson A., Clancy R. The epidemiology of malaria in a population surrounding Madang, Papua New Guinea. Am J Trop Med Hyg. 1986 Jan;35(1):3–15. doi: 10.4269/ajtmh.1986.35.3. [DOI] [PubMed] [Google Scholar]
- Crane G., Bhatia K., Honeyman M., Doran T., Messel N., Hakos G., Tarlinton D., Amos D. B., Bashir H. HLA studies of Highland and Coastal New Guineans. Hum Immunol. 1985 Apr;12(4):247–260. doi: 10.1016/0198-8859(85)90340-4. [DOI] [PubMed] [Google Scholar]
- Del Giudice G., Cooper J. A., Merino J., Verdini A. S., Pessi A., Togna A. R., Engers H. D., Corradin G., Lambert P. H. The antibody response in mice to carrier-free synthetic polymers of Plasmodium falciparum circumsporozoite repetitive epitope is I-Ab-restricted: possible implications for malaria vaccines. J Immunol. 1986 Nov 1;137(9):2952–2955. [PubMed] [Google Scholar]
- Del Giudice G., Engers H. D., Tougne C., Biro S. S., Weiss N., Verdini A. S., Pessi A., Degremont A. A., Freyvogel T. A., Lambert P. H. Antibodies to the repetitive epitope of Plasmodium falciparum circumsporozoite protein in a rural Tanzanian community: a longitudinal study of 132 children. Am J Trop Med Hyg. 1987 Mar;36(2):203–212. doi: 10.4269/ajtmh.1987.36.203. [DOI] [PubMed] [Google Scholar]
- Good M. F., Berzofsky J. A., Maloy W. L., Hayashi Y., Fujii N., Hockmeyer W. T., Miller L. H. Genetic control of the immune response in mice to a Plasmodium falciparum sporozoite vaccine. Widespread nonresponsiveness to single malaria T epitope in highly repetitive vaccine. J Exp Med. 1986 Aug 1;164(2):655–660. doi: 10.1084/jem.164.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Miller L. H., Kumar S., Quakyi I. A., Keister D., Adams J. H., Moss B., Berzofsky J. A., Carter R. Limited immunological recognition of critical malaria vaccine candidate antigens. Science. 1988 Oct 28;242(4878):574–577. doi: 10.1126/science.2902690. [DOI] [PubMed] [Google Scholar]
- Good M. F., Pombo D., Maloy W. L., de la Cruz V. F., Miller L. H., Berzofsky J. A. Parasite polymorphism present within minimal T cell epitopes of Plasmodium falciparum circumsporozoite protein. J Immunol. 1988 Mar 1;140(5):1645–1650. [PubMed] [Google Scholar]
- Good M. F., Pombo D., Quakyi I. A., Riley E. M., Houghten R. A., Menon A., Alling D. W., Berzofsky J. A., Miller L. H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves P. M., Burkot T. R., Carter R., Cattani J. A., Lagog M., Parker J., Brabin B. J., Gibson F. D., Bradley D. J., Alpers M. P. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology. 1988 Apr;96(Pt 2):251–263. doi: 10.1017/s003118200005825x. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Carter R., Burkot T. R., Quakyi I. A., Kumar N. Antibodies to Plasmodium falciparum gamete surface antigens in Papua New Guinea sera. Parasite Immunol. 1988 Mar;10(2):209–218. doi: 10.1111/j.1365-3024.1988.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Wirtz R. A., Carter R., Burkot T. R., Looker M., Targett G. A. Naturally occurring antibodies to an epitope on Plasmodium falciparum gametes detected by monoclonal antibody-based competitive enzyme-linked immunosorbent assay. Infect Immun. 1988 Nov;56(11):2818–2821. doi: 10.1128/iai.56.11.2818-2821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S. L., Wistar R., Jr, Ballou W. R., Hollingdale M. R., Wirtz R. A., Schneider I., Marwoto H. A., Hockmeyer W. T. Immunity to malaria and naturally acquired antibodies to the circumsporozoite protein of Plasmodium falciparum. N Engl J Med. 1986 Sep 4;315(10):601–606. doi: 10.1056/NEJM198609043151001. [DOI] [PubMed] [Google Scholar]
- Kumar S., Miller L. H., Quakyi I. A., Keister D. B., Houghten R. A., Maloy W. L., Moss B., Berzofsky J. A., Good M. F. Cytotoxic T cells specific for the circumsporozoite protein of Plasmodium falciparum. Nature. 1988 Jul 21;334(6179):258–260. doi: 10.1038/334258a0. [DOI] [PubMed] [Google Scholar]
- Nardin E. H., Nussenzweig R. S., McGregor I. A., Bryan J. H. Antibodies to sporozoites: their frequent occurrence in individuals living in an area of hyperendemic malaria. Science. 1979 Nov 2;206(4418):597–599. doi: 10.1126/science.386511. [DOI] [PubMed] [Google Scholar]
- Rener J., Graves P. M., Carter R., Williams J. L., Burkot T. R. Target antigens of transmission-blocking immunity on gametes of plasmodium falciparum. J Exp Med. 1983 Sep 1;158(3):976–981. doi: 10.1084/jem.158.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield L., Villaquiran J., Ferreira A., Schellekens H., Nussenzweig R., Nussenzweig V. Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites. Nature. 1987 Dec 17;330(6149):664–666. doi: 10.1038/330664a0. [DOI] [PubMed] [Google Scholar]
- Vermeulen A. N., Ponnudurai T., Beckers P. J., Verhave J. P., Smits M. A., Meuwissen J. H. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med. 1985 Nov 1;162(5):1460–1476. doi: 10.1084/jem.162.5.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]


