Abstract
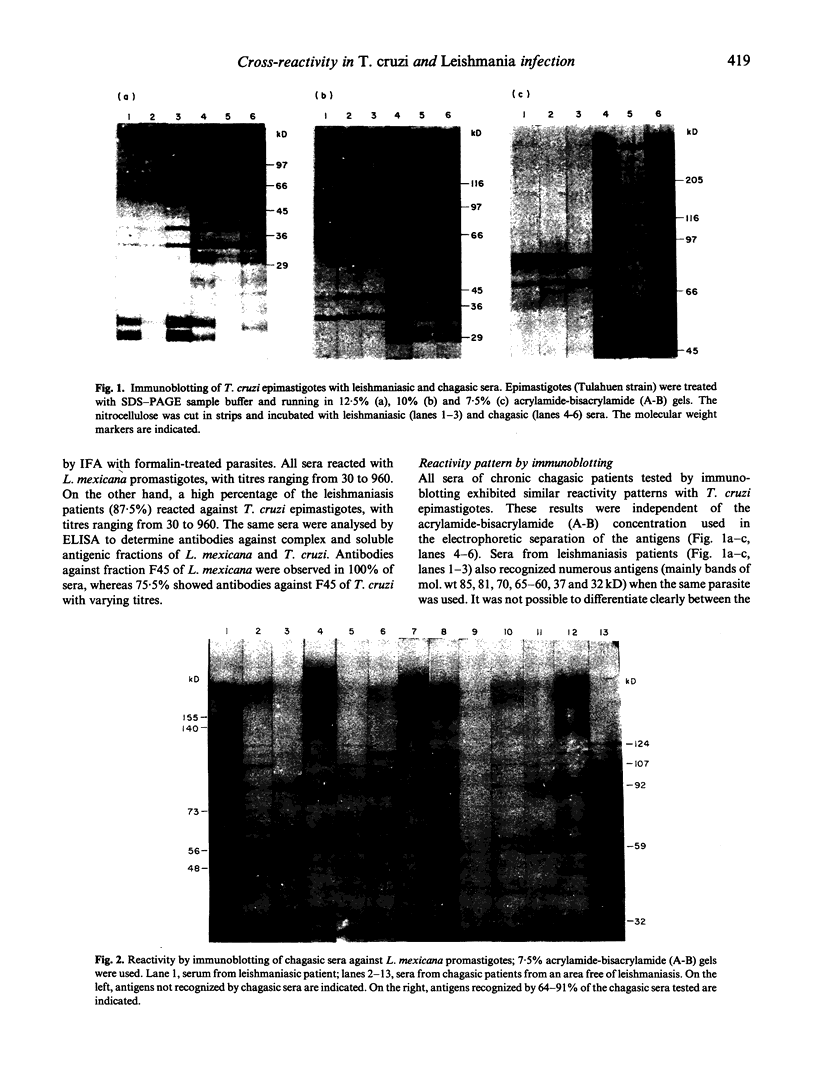
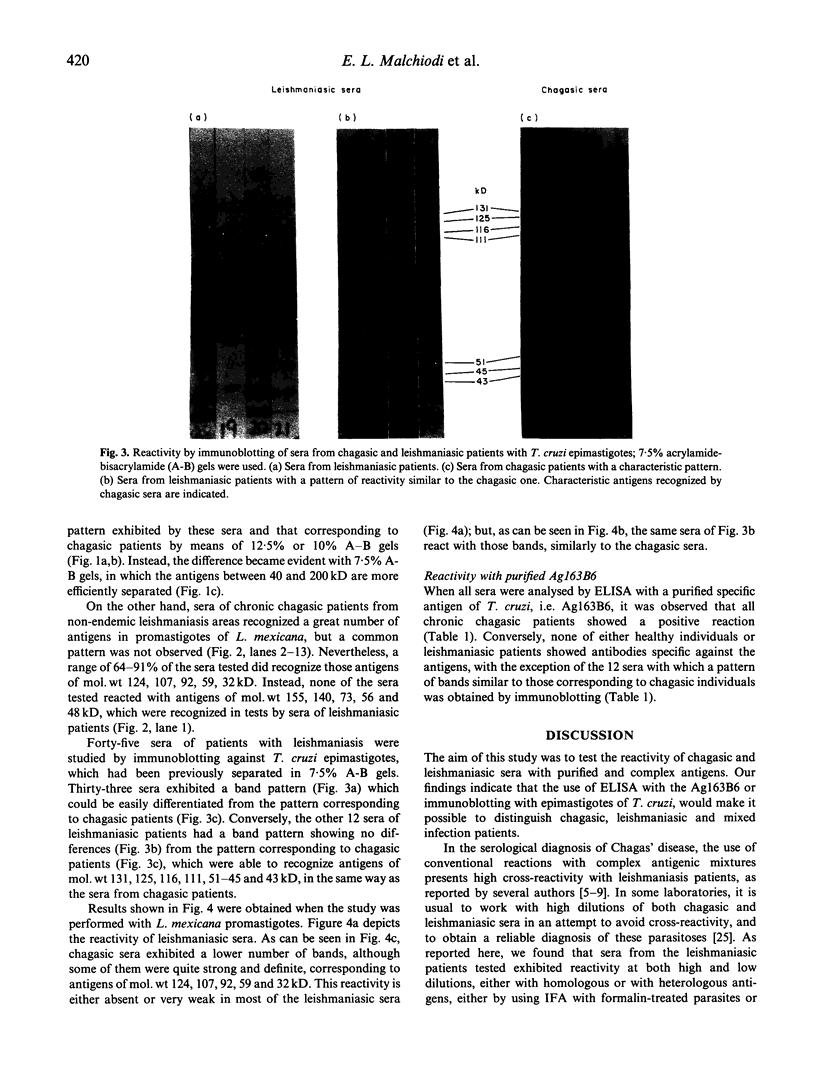
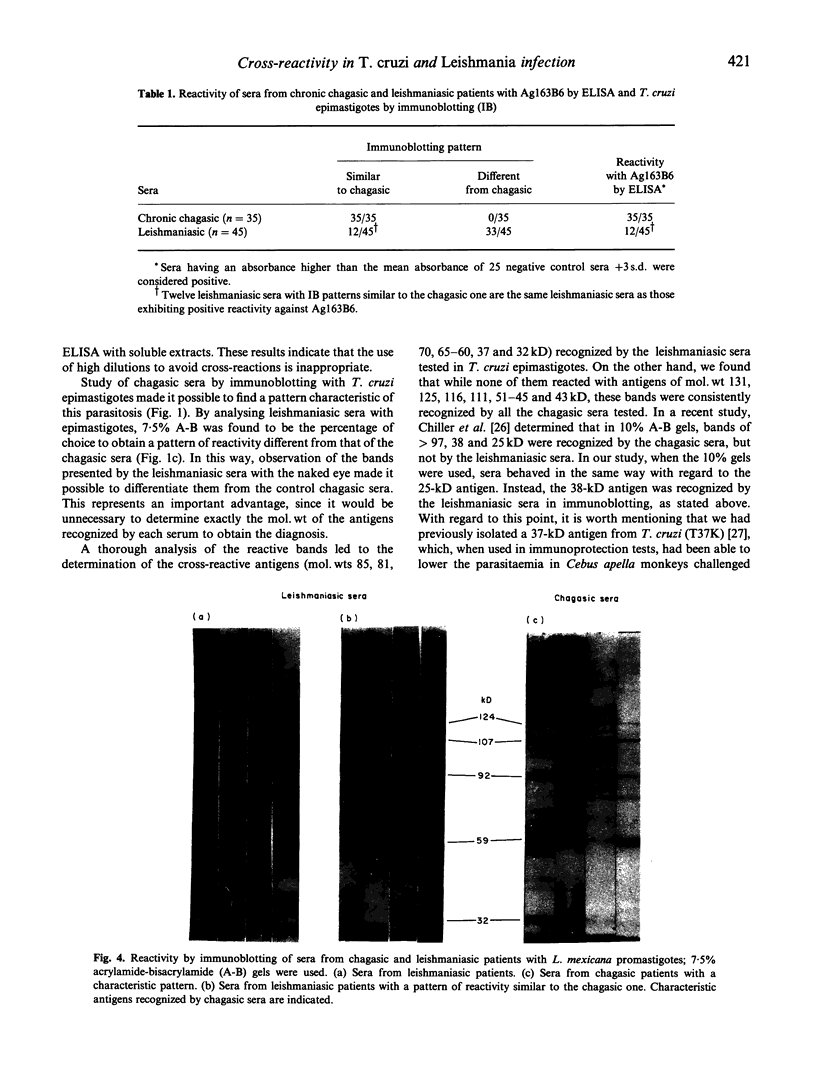
Results of our studies on the reactivity of chagasic and leishmaniasic sera with the purified T. cruzi-specific antigen 163B6, as assessed by ELISA, and with complex antigenic mixtures from T. cruzi and Leishmania mexicana, by immunoblotting, are presented here. Our objective was to identify the antigens responsible for the exhibited cross-reactivity between trypanosomiasis and leishmaniasis, and to find a specific reactivity pattern corresponding to each parasitosis. In spite of the high cross-reactivity observed with the immunoblotting, the use of 7.5% A-B gels made it possible to identify a characteristic pattern for each parasitosis, that could be distinguished by the naked eye. The characteristic pattern corresponding to chagasic patients was ascribed to reactivity with T. cruzi bands of mol. wts 131, 125, 116, 111, 51-45 and 43 kD, that were not recognized by leishmaniasic sera. Trypanosoma cruzi antigens of mol. wts 85, 81, 70, 65-60, 37 and 32 kD were considered as crossing antigens, since they were recognized by leishmaniasis sera. With L. mexicana, most of the chagasic patients presented reaction with antigen of mol. wts 124, 107, 92, 59 and 32 kD, while bands of mol. wts 155, 140, 73, 56 and 48 kD were recognized only by leishmaniasic sera. In this study we found 12 out of 45 sera of patients with leishmaniasis, from a region endemic for both parasitoses, which exhibited a pattern of bands very similar to those corresponding to chagasic individuals, strongly suggesting a mixed infection. This hypothesis was verified by using a purified specific antigen of T. cruzi, Ag163B6, which would be the major cysteine proteinase of this specie (cruzipain). By ELISA, these 12 sera showed a positive reaction with this purified antigen, as those of chagasic patients, thus leading to the confirmation of the presence of a mixed infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez M., Cerisola J. A., Rohwedder R. W. Test de inmunofluorescencia para diagnóstico de la enfermedad de Chagas. Bol Chil Parasitol. 1968 Jan-Jun;23(1):4–8. [PubMed] [Google Scholar]
- Araujo F. G. Analysis of Trypanosoma cruzi antigens bound by specific antibodies and by antibodies to related trypanosomatids. Infect Immun. 1986 Jul;53(1):179–185. doi: 10.1128/iai.53.1.179-185.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi E., Cazzulo J. J. Digestion of human immunoglobulin G by the major cysteine proteinase (cruzipain) from Trypanosoma cruzi. FEMS Microbiol Lett. 1990 Aug;58(3):337–341. doi: 10.1111/j.1574-6968.1990.tb14000.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brener Z. Recent developments in the field of Chagas' disease. Bull World Health Organ. 1982;60(4):463–473. [PMC free article] [PubMed] [Google Scholar]
- Camargo M. E., Rebonato C. Cross-reactivity in fluorescence tests for Trypanosoma and Leishmania antibodies. A simple inhibition procedure to ensure specific results. Am J Trop Med Hyg. 1969 Jul;18(4):500–505. doi: 10.4269/ajtmh.1969.18.500. [DOI] [PubMed] [Google Scholar]
- Carbonetto C. H., Malchiodi E. L., Chiaramonte M., Durante de Isola E., Fossati C. A., Margni R. A. Isolation of a Trypanosoma cruzi antigen by affinity chromatography with a monoclonal antibody. Preliminary evaluation of its possible applications in serological tests. Clin Exp Immunol. 1990 Oct;82(1):93–96. doi: 10.1111/j.1365-2249.1990.tb05409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiller T. M., Samudio M. A., Zoulek G. IgG antibody reactivity with Trypanosoma cruzi and Leishmania antigens in sera of patients with Chagas' disease and leishmaniasis. Am J Trop Med Hyg. 1990 Dec;43(6):650–656. doi: 10.4269/ajtmh.1990.43.650. [DOI] [PubMed] [Google Scholar]
- Desjeux P., Aranda E., Aliaga O., Mollinedo S. Human visceral leishmaniasis in Bolivia: first proven autochthonous case from 'Los Yungas'. Trans R Soc Trop Med Hyg. 1983;77(6):851–852. doi: 10.1016/0035-9203(83)90305-x. [DOI] [PubMed] [Google Scholar]
- Guimarães M. C., Celeste B. J., de Castilho E. A., Mineo J. R., Diniz J. M. Immunoenzymatic assy (ELISA) in mucocutaneous leishmaniasis, kala-azar, and Chagas' disease: an epimastigote Trypanosoma cruzi antigen able to distinguish between anti-Trypanosoma and anti-Leishmania antibodies. Am J Trop Med Hyg. 1981 Sep;30(5):942–947. doi: 10.4269/ajtmh.1981.30.942. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Serodiagnostic assay for visceral leishmaniasis employing monoclonal antibodies. Trans R Soc Trop Med Hyg. 1987;81(4):587–594. doi: 10.1016/0035-9203(87)90418-4. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Zalis M. Purification of two Leishmania donovani membrane proteins recognized by sera from patients with visceral leishmaniasis. Mol Biochem Parasitol. 1988 Jan 1;27(1):53–62. doi: 10.1016/0166-6851(88)90024-2. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Zalis M. Use of purified parasite proteins from Leishmania donovani for the rapid serodiagnosis of visceral leishmaniasis. J Infect Dis. 1988 Jun;157(6):1212–1220. doi: 10.1093/infdis/157.6.1212. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy Yeyati P., Bonnefoy S., Mirkin G., Debrabant A., Lafon S., Panebra A., Gonzalez-Cappa E., Dedet J. P., Hontebeyrie-Joskowicz M., Levin M. J. The 70-kDa heat-shock protein is a major antigenic determinant in human Trypanosoma cruzi/Leishmania braziliensis braziliensis mixed infection. Immunol Lett. 1992 Jan;31(1):27–33. doi: 10.1016/0165-2478(92)90006-a. [DOI] [PubMed] [Google Scholar]
- Luquetti A. O. Use of Trypanosoma cruzi defined proteins for diagnosis--Multicentre trial. Serological and technical aspects. Mem Inst Oswaldo Cruz. 1990 Oct-Dec;85(4):497–505. doi: 10.1590/s0074-02761990000400021. [DOI] [PubMed] [Google Scholar]
- Malchiodi E. L., Carbonetto C. H., Grana D., Eiguchi de Palmero K., Chiaramonte M. G., Falasca C. A., Margni R. A. Immune response against Trypanosoma cruzi antigens in Cebus apella monkeys. Trop Med Parasitol. 1993 Jun;44(2):86–90. [PubMed] [Google Scholar]
- Malchiodi E. L., Carbonetto C. H., Margni R. A. Isolation-characterization and preliminary immunological studies of a Trypanosoma cruzi antigenic component. Medicina (B Aires) 1987;47(5):509–515. [PubMed] [Google Scholar]
- Malchiodi E. L., Chiaramonte M. G., Martínez J. A., Zwirner N. W., Margni R. A., Cazzulo J. J. Identity of the major cysteine proteinase (cruzipain) from Trypanosoma cruzi and an antigen (Ag163B6) isolated with a monoclonal antibody. Immunol Lett. 1993 Jan;35(1):59–62. doi: 10.1016/0165-2478(93)90148-u. [DOI] [PubMed] [Google Scholar]
- Martinez J., Campetella O., Frasch A. C., Cazzulo J. J. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is antigenic in human infections. Infect Immun. 1991 Nov;59(11):4275–4277. doi: 10.1128/iai.59.11.4275-4277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel J. P., Howard R. J., Dvorak J. A. Identification and analysis of epimastigote surface and metabolic proteins in Trypanosoma cruzi. Mol Biochem Parasitol. 1986 May;19(2):183–194. doi: 10.1016/0166-6851(86)90123-4. [DOI] [PubMed] [Google Scholar]
- Schattschneider W., Lopes E. R., De Alencar J. E., Bienzle U., Feldmeier H. A comparative study of four serological methods for diagnosis of acute and chronic Chagas' disease in Brazilian patients. Trop Geogr Med. 1992 Jul;44(3):210–218. [PubMed] [Google Scholar]
- Souto-Padrón T., Campetella O. E., Cazzulo J. J., de Souza W. Cysteine proteinase in Trypanosoma cruzi: immunocytochemical localization and involvement in parasite-host cell interaction. J Cell Sci. 1990 Jul;96(Pt 3):485–490. doi: 10.1242/jcs.96.3.485. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton B. C., Chinel L. V., Eguia y Eguia O. Onset of espundia after many years of occult infection with Leishmania braziliensis. Am J Trop Med Hyg. 1973 Nov;22(6):696–698. doi: 10.4269/ajtmh.1973.22.696. [DOI] [PubMed] [Google Scholar]