Abstract
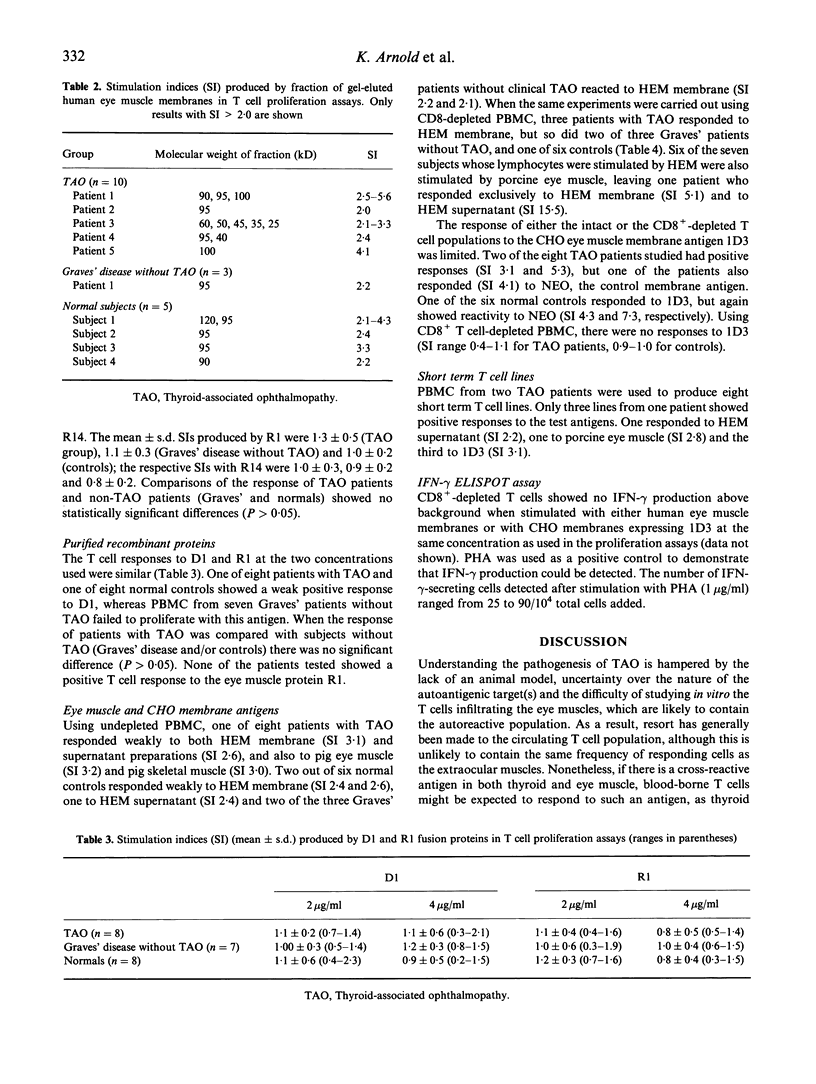
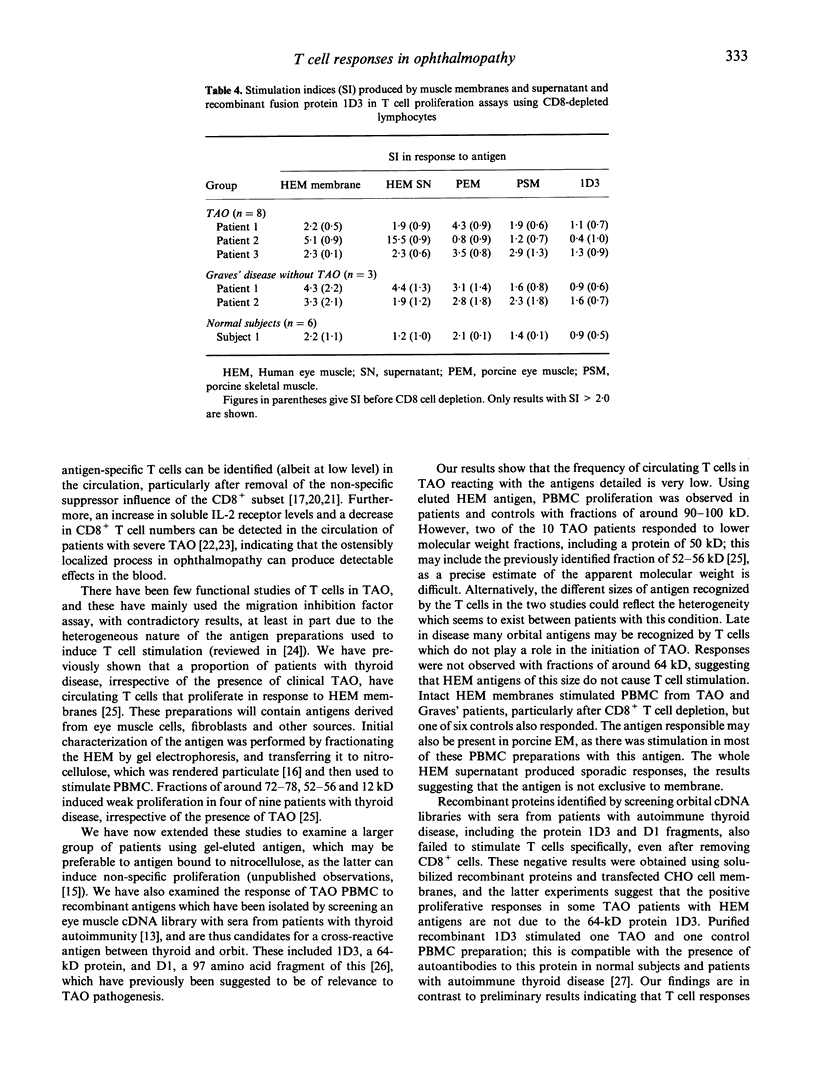
Thyroid-associated ophthalmopathy (TAO) is most likely to be a T cell-mediated disease, in which cytokines released in the extraocular muscles activate fibroblasts, increasing glycosaminoglycan production. The nature of the orbital antigen recognized by the infiltrating T cells is unclear, although it is possible that there is cross-reactivity between this and a thyroid autoantigen to explain the close association with thyroid autoimmunity. We have tested the ability of human and porcine eye muscle antigen preparations to stimulate proliferation of circulating T cells from healthy subjects and patients with TAO or Graves' disease without clinical TAO. Occasional responses were seen, particularly after depletion of CD8+ T cells, and two out of 10 TAO patients responded to eye muscle proteins of 25-50 kD after fractionation of antigens on gels and subsequent elution. There was no disease-specific response of T cells to R1, R14, D1 and 1D3, recombinant proteins identified from screening an eye muscle cDNA library with sera from patients with autoimmune thyroid disease. We have also found that interferon-gamma (IFN-gamma) production by T cells from TAO patients was not stimulated by eye muscle membrane antigens or by 1D3. These results suggest that the frequency of circulating T cells responding to eye muscle antigens in TAO is low, and that several candidate orbital antigens, including the 64-kD protein 1D3, are unlikely to be important T cell autoantigens in this condition.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Zeid C., Filley E., Steele J., Rook G. A. A simple new method for using antigens separated by polyacrylamide gel electrophoresis to stimulate lymphocytes in vitro after converting bands cut from Western blots into antigen-bearing particles. J Immunol Methods. 1987 Apr 2;98(1):5–10. doi: 10.1016/0022-1759(87)90429-7. [DOI] [PubMed] [Google Scholar]
- Czerkinsky C., Andersson G., Ekre H. P., Nilsson L. A., Klareskog L., Ouchterlony O. Reverse ELISPOT assay for clonal analysis of cytokine production. I. Enumeration of gamma-interferon-secreting cells. J Immunol Methods. 1988 May 25;110(1):29–36. doi: 10.1016/0022-1759(88)90079-8. [DOI] [PubMed] [Google Scholar]
- Dong Q., Ludgate M., Vassart G. Cloning and sequencing of a novel 64-kDa autoantigen recognized by patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1991 Jun;72(6):1375–1381. doi: 10.1210/jcem-72-6-1375. [DOI] [PubMed] [Google Scholar]
- Elisei R., Weightman D., Kendall-Taylor P., Vassart G., Ludgate M. Muscle autoantigens in thyroid associated ophthalmopathy: the limits of molecular genetics. J Endocrinol Invest. 1993 Jul-Aug;16(7):533–540. doi: 10.1007/BF03348900. [DOI] [PubMed] [Google Scholar]
- Ewins D. L., Barnett P. S., Ratanachaiyavong S., Sharrock C., Lanchbury J., McGregor A. M., Banga J. P. Antigen-specific T cell recognition of affinity-purified and recombinant thyroid peroxidase in autoimmune thyroid disease. Clin Exp Immunol. 1992 Oct;90(1):93–98. doi: 10.1111/j.1365-2249.1992.tb05838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuma N., McLachlan S. M., Rapoport B., Goodacre J., Middleton S. L., Phillips D. I., Pegg C. A., Rees Smith B. Thyroid autoantigens and human T cell responses. Clin Exp Immunol. 1990 Nov;82(2):275–283. doi: 10.1111/j.1365-2249.1990.tb05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Detection and localization of cytokine immunoreactivity in retro-ocular connective tissue in Graves' ophthalmopathy. Eur J Clin Invest. 1993 Jan;23(1):10–17. doi: 10.1111/j.1365-2362.1993.tb00712.x. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Bahn R. S. Graves' immunoglobulins and cytokines stimulate the expression of intercellular adhesion molecule-1 (ICAM-1) in cultured Graves' orbital fibroblasts. Eur J Clin Invest. 1992 Aug;22(8):529–537. doi: 10.1111/j.1365-2362.1992.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Smith T. J., Gorman C. A., Bahn R. S. Increased induction of HLA-DR by interferon-gamma in cultured fibroblasts derived from patients with Graves' ophthalmopathy and pretibial dermopathy. J Clin Endocrinol Metab. 1991 Aug;73(2):307–313. doi: 10.1210/jcem-73-2-307. [DOI] [PubMed] [Google Scholar]
- Heufelder A. E., Wenzel B. E., Gorman C. A., Bahn R. S. Detection, cellular localization, and modulation of heat shock proteins in cultured fibroblasts from patients with extrathyroidal manifestations of Graves' disease. J Clin Endocrinol Metab. 1991 Oct;73(4):739–745. doi: 10.1210/jcem-73-4-739. [DOI] [PubMed] [Google Scholar]
- Kroll A. J., Kuwabara T. Dysthyroid ocular myopathy. Anatomy, histology, and electron microscopy. Arch Ophthalmol. 1966 Aug;76(2):244–247. doi: 10.1001/archopht.1966.03850010246017. [DOI] [PubMed] [Google Scholar]
- Metcalfe R. A., Weetman A. P. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: possible role in thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf) 1994 Jan;40(1):67–72. doi: 10.1111/j.1365-2265.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]
- Persidis A., Harcombe A. A. Simultaneous electroelution of proteins from denaturing or native gels into a well matrix. Anal Biochem. 1992 Feb 14;201(1):185–189. doi: 10.1016/0003-2697(92)90193-b. [DOI] [PubMed] [Google Scholar]
- Prummel M. F., Wiersinga W. M., Van der Gaag R., Mourits M. P., Koornneef L. Soluble IL-2 receptor levels in patients with Graves' ophthalmopathy. Clin Exp Immunol. 1992 Jun;88(3):405–409. doi: 10.1111/j.1365-2249.1992.tb06462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roep B. O., Kallan A. A., Hazenbos W. L., Bruining G. J., Bailyes E. M., Arden S. D., Hutton J. C., de Vries R. R. T-cell reactivity to 38 kD insulin-secretory-granule protein in patients with recent-onset type 1 diabetes. Lancet. 1991 Jun 15;337(8755):1439–1441. doi: 10.1016/0140-6736(91)93127-u. [DOI] [PubMed] [Google Scholar]
- Ross P. V., Koenig R. J., Arscott P., Ludgate M., Waier M., Nelson C. C., Kaplan M. M., Baker J. R., Jr Tissue specificity and serologic reactivity of an autoantigen associated with autoimmune thyroid disease. J Clin Endocrinol Metab. 1993 Aug;77(2):433–438. doi: 10.1210/jcem.77.2.8345048. [DOI] [PubMed] [Google Scholar]
- Salvi M., Zhang Z. G., Haegert D., Woo M., Liberman A., Cadarso L., Wall J. R. Patients with endocrine ophthalmopathy not associated with overt thyroid disease have multiple thyroid immunological abnormalities. J Clin Endocrinol Metab. 1990 Jan;70(1):89–94. doi: 10.1210/jcem-70-1-89. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Bahn R. S., Gorman C. A., Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991 May;72(5):1169–1171. doi: 10.1210/jcem-72-5-1169. [DOI] [PubMed] [Google Scholar]
- Tallstedt L., Norberg R. Immunohistochemical staining of normal and Graves' extraocular muscle. Invest Ophthalmol Vis Sci. 1988 Feb;29(2):175–184. [PubMed] [Google Scholar]
- Tandon N., Freeman M., Weetman A. P. T cell responses to synthetic thyroid peroxidase peptides in autoimmune thyroid disease. Clin Exp Immunol. 1991 Oct;86(1):56–60. doi: 10.1111/j.1365-2249.1991.tb05773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyutyunikov A., Raikow R. B., Kennerdell J. S., Kazim M., Dalbow M. H., Scalise D. Re-examination of peripheral blood T cell subsets in dysthyroid orbitopathy. Invest Ophthalmol Vis Sci. 1992 Jun;33(7):2299–2303. [PubMed] [Google Scholar]
- Van Dyk H. J. Orbital Graves' disease. A modification of the "NO SPECS" classification. Ophthalmology. 1981 Jun;88(6):479–483. [PubMed] [Google Scholar]
- Wall J. R., Bernard N., Boucher A., Salvi M., Zhang Z. G., Kennerdell J., Tyutyunikov A., Genovese C. Pathogenesis of thyroid-associated ophthalmopathy: an autoimmune disorder of the eye muscle associated with Graves' hyperthyroidism and Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1993 Jul;68(1):1–8. doi: 10.1006/clin.1993.1087. [DOI] [PubMed] [Google Scholar]
- Weetman A. P. Autologous CD8-positive cells suppress T cell proliferation in response to thyroid antigens in Hashimoto's thyroiditis. Clin Immunol Immunopathol. 1989 May;51(2):303–310. doi: 10.1016/0090-1229(89)90028-7. [DOI] [PubMed] [Google Scholar]
- Weetman A. P., Cohen S., Gatter K. C., Fells P., Shine B. Immunohistochemical analysis of the retrobulbar tissues in Graves' ophthalmopathy. Clin Exp Immunol. 1989 Feb;75(2):222–227. [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., Fells P., Shine B. T and B cell reactivity to extraocular and skeletal muscle in Graves' ophthalmopathy. Br J Ophthalmol. 1989 May;73(5):323–327. doi: 10.1136/bjo.73.5.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P. The role of T lymphocytes in thyroid-associated ophthalmopathy. Autoimmunity. 1992;13(1):69–73. doi: 10.3109/08916939209014637. [DOI] [PubMed] [Google Scholar]
- de Carli M., D'Elios M. M., Mariotti S., Marcocci C., Pinchera A., Ricci M., Romagnani S., del Prete G. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves' ophthalmopathy. J Clin Endocrinol Metab. 1993 Nov;77(5):1120–1124. doi: 10.1210/jcem.77.5.8077301. [DOI] [PubMed] [Google Scholar]



