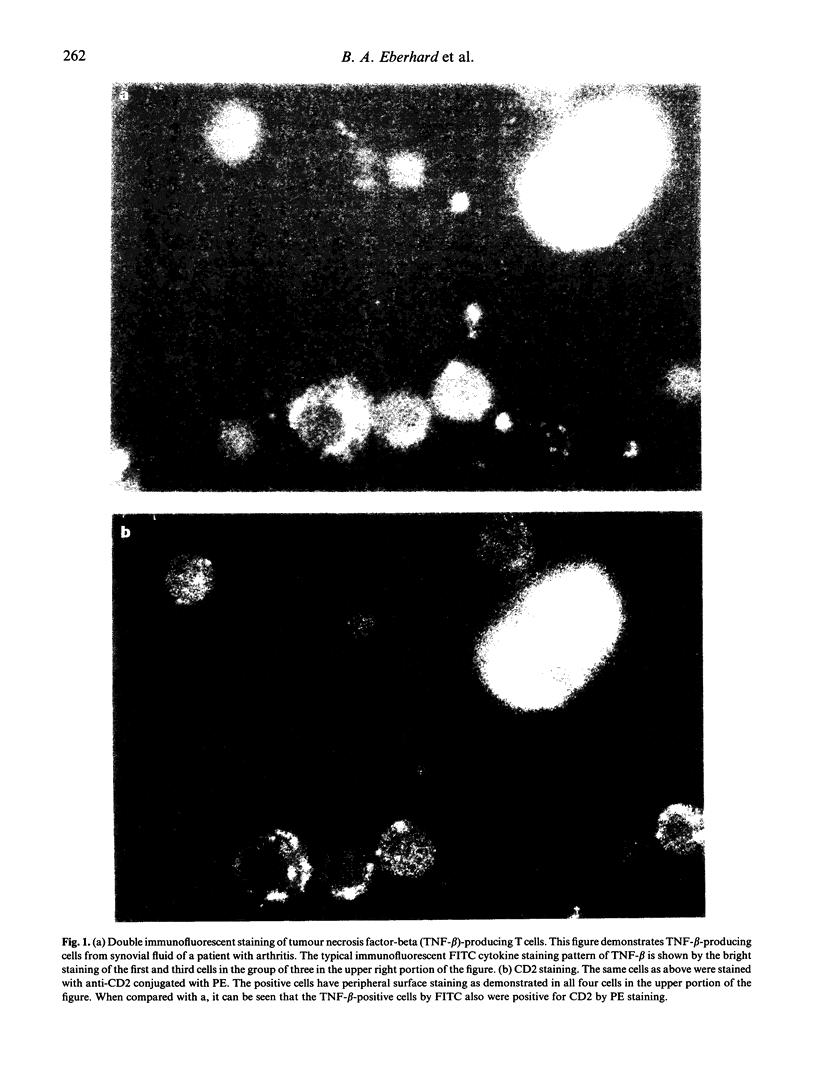
Abstract
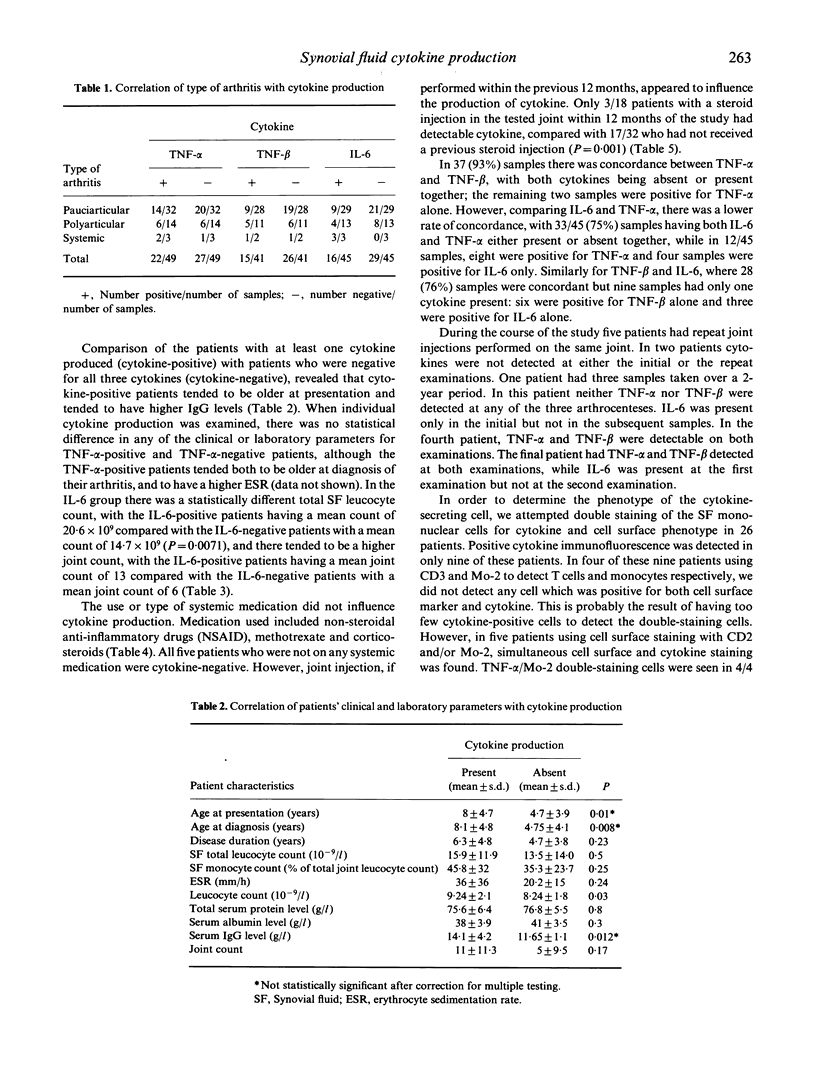
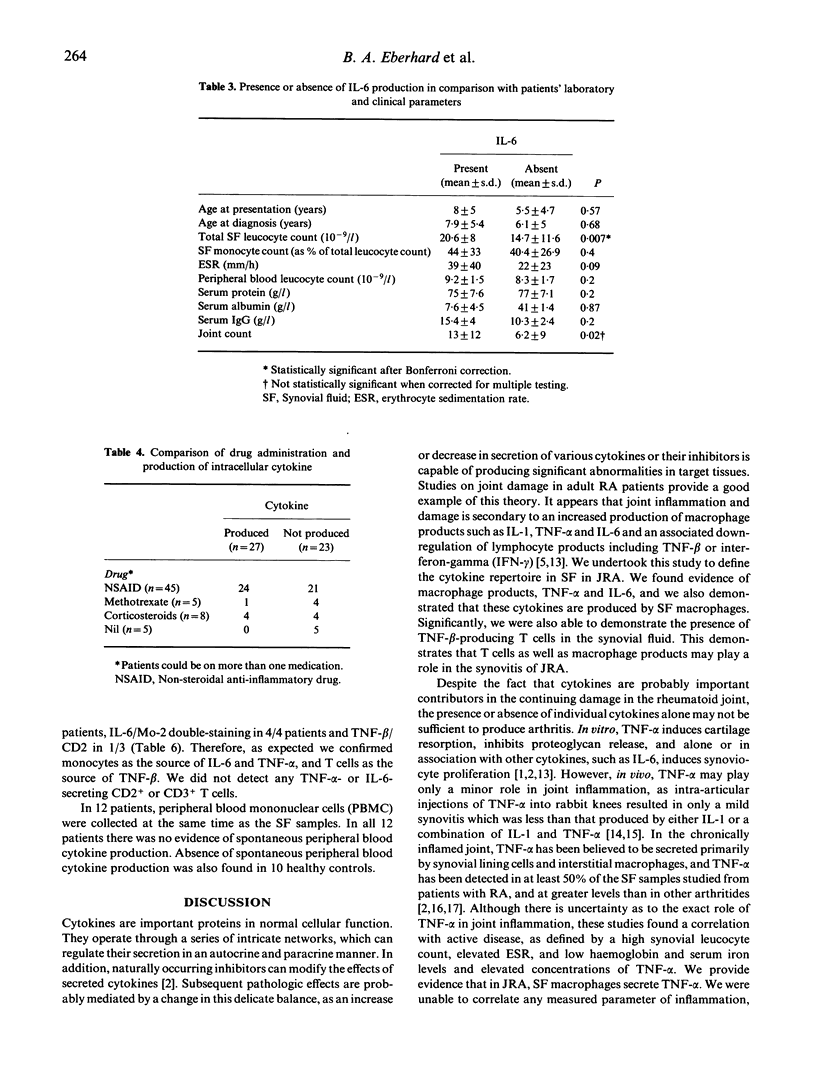
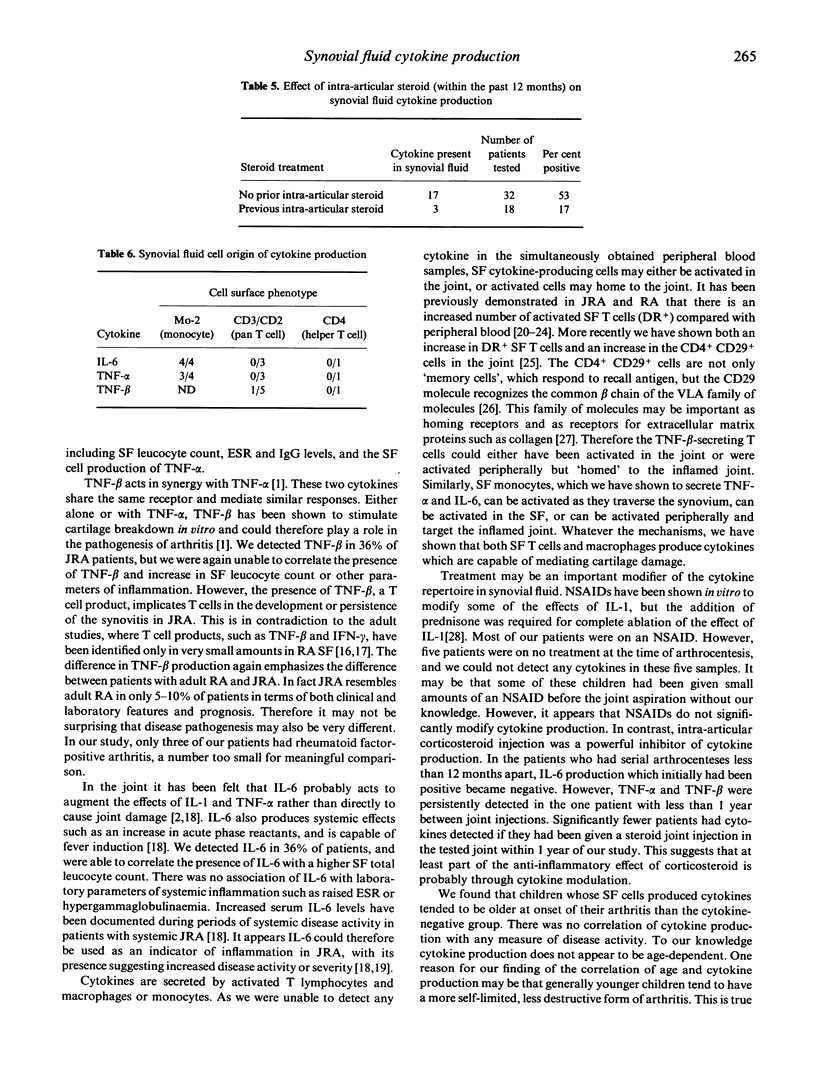
The production of tumour necrosis factor-alpha (TNF-alpha), TNF-beta and IL-6 in synovial fluid was studied in 50 samples of synovial fluid from 44 children with juvenile rheumatoid arthritis (JRA) by identifying cytokine production at a single-cell level. Post Ficoll-separated synovial fluid mononuclear cells were permeabilized and then intracellular TNF-alpha, TNF-beta and IL-6 protein production was examined using indirect immunofluorescence and murine anti-cytokine MoAbs. All three cytokines were measured in 37 of the 50 samples. In 25 of the 37 samples there was complete concordance; all three cytokines were present in six and absent in 19 samples. At least one cytokine was present in 27/50 (54%) of synovial fluid samples. Overall, TNF-alpha was detected in 22/49 (45%) samples, TNF-beta in 15/41 (37%) and IL-6 in 16/45 (36%) samples. Five patients had serial arthrocentesis, and in these samples there were two patients who had initially positive cytokine production, which on subsequent measurement was negative; in the other three patients there was no change from the previous cytokine production. We provide evidence that synovial fluid mononuclear cells produce monocyte and T cell cytokines in JRA. These findings suggest a role for both T cell and macrophage products in the pathogenesis of JRA, and the potential for modulation of cytokine production as a target for therapeutic intervention.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990 Dec;86(6):1790–1798. doi: 10.1172/JCI114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson U., Sander B., Andersson J., Möller G. Concomitant production of different lymphokines in activated T cells. Eur J Immunol. 1988 Dec;18(12):2081–2084. doi: 10.1002/eji.1830181232. [DOI] [PubMed] [Google Scholar]
- Arend W. P., Dayer J. M. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990 Mar;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990 Mar 1;75(5):1037–1050. [PubMed] [Google Scholar]
- Bergroth V., Konttinen Y. T., Pelkonen P., Haapala M., Haapasaari J., Nordström D., Kunnamo I., Friman C. Synovial fluid lymphocytes in different subtypes of juvenile rheumatoid arthritis. Arthritis Rheum. 1988 Jun;31(6):780–783. doi: 10.1002/art.1780310613. [DOI] [PubMed] [Google Scholar]
- Brewer E. J., Jr, Bass J., Baum J., Cassidy J. T., Fink C., Jacobs J., Hanson V., Levinson J. E., Schaller J., Stillman J. S. Current proposed revision of JRA Criteria. JRA Criteria Subcommittee of the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Section of The Arthritis Foundation. Arthritis Rheum. 1977 Mar;20(2 Suppl):195–199. [PubMed] [Google Scholar]
- Brozik M., Rosztóczy I., Merétey K., Bálint G., Gaál M., Balogh Z., Bart M., Mituszova M., Velics V., Falus A. Interleukin 6 levels in synovial fluids of patients with different arthritides: correlation with local IgM rheumatoid factor and systemic acute phase protein production. J Rheumatol. 1992 Jan;19(1):63–68. [PubMed] [Google Scholar]
- Campbell I. K., Piccoli D. S., Roberts M. J., Muirden K. D., Hamilton J. A. Effects of tumor necrosis factor alpha and beta on resorption of human articular cartilage and production of plasminogen activator by human articular chondrocytes. Arthritis Rheum. 1990 Apr;33(4):542–552. doi: 10.1002/art.1780330412. [DOI] [PubMed] [Google Scholar]
- Dasgupta B., Corkill M., Kirkham B., Gibson T., Panayi G. Serial estimation of interleukin 6 as a measure of systemic disease in rheumatoid arthritis. J Rheumatol. 1992 Jan;19(1):22–25. [PubMed] [Google Scholar]
- De Benedetti F., Robbioni P., Massa M., Viola S., Albani S., Martini A. Serum interleukin-6 levels and joint involvement in polyarticular and pauciarticular juvenile chronic arthritis. Clin Exp Rheumatol. 1992 Sep-Oct;10(5):493–498. [PubMed] [Google Scholar]
- De Maria A. F., Malnati M. S., Poggi A., Pende D., Cottafava F., Moretta L. Clonal analysis of joint fluid T lymphocytes in patients with juvenile rheumatoid arthritis. J Rheumatol. 1990 Aug;17(8):1073–1078. [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- Firestein G. S., Xu W. D., Townsend K., Broide D., Alvaro-Gracia J., Glasebrook A., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988 Nov 1;168(5):1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Zvaifler N. J. How important are T cells in chronic rheumatoid synovitis? Arthritis Rheum. 1990 Jun;33(6):768–773. doi: 10.1002/art.1780330602. [DOI] [PubMed] [Google Scholar]
- Førre O., Dobloug J. H., Natvig J. B. Augmented numbers of HLA-DR-positive T lymphocytes in the synovial fluid and synovial tissue of patients with rheumatoid arthritis and juvenile rheumatoid arthritis: in vivo-activated T lymphocytes are potent stimulators in the mixed lymphocyte reaction. Scand J Immunol. 1982 Feb;15(2):227–231. doi: 10.1111/j.1365-3083.1982.tb00643.x. [DOI] [PubMed] [Google Scholar]
- Førre O., Thoen J., Dobloug J. H., Egeland T., Kvien T. K., Mellbye O. J., Natvig J. B. Detection of T-lymphocyte subpopulation in the peripheral blood and the synovium of patients with rheumatoid arthritis and juvenile rheumatoid arthritis using monoclonal antibodies. Scand J Immunol. 1982 Feb;15(2):221–226. doi: 10.1111/j.1365-3083.1982.tb00642.x. [DOI] [PubMed] [Google Scholar]
- Henderson B., Pettipher E. R. Arthritogenic actions of recombinant IL-1 and tumour necrosis factor alpha in the rabbit: evidence for synergistic interactions between cytokines in vivo. Clin Exp Immunol. 1989 Feb;75(2):306–310. [PMC free article] [PubMed] [Google Scholar]
- Holt I., Cooper R. G., Denton J., Meager A., Hopkins S. J. Cytokine inter-relationships and their association with disease activity in arthritis. Br J Rheumatol. 1992 Nov;31(11):725–733. doi: 10.1093/rheumatology/31.11.725. [DOI] [PubMed] [Google Scholar]
- Hopkins S. J., Meager A. Cytokines in synovial fluid: II. The presence of tumour necrosis factor and interferon. Clin Exp Immunol. 1988 Jul;73(1):88–92. [PMC free article] [PubMed] [Google Scholar]
- Lane N. E., Williams R. J., 3rd, Schurman D. J., Smith R. L. Inhibition of interleukin 1 induced chondrocyte protease activity by a corticosteroid and a nonsteroidal antiinflammatory drug. J Rheumatol. 1992 Jan;19(1):135–139. [PubMed] [Google Scholar]
- Nakao H., Eguchi K., Kawakami A., Migita K., Otsubo T., Ueki Y., Shimomura C., Tezuka H., Matsunaga M., Maeda K. Phenotypic characterization of lymphocytes infiltrating synovial tissue from patients with rheumatoid arthritis: analysis of lymphocytes isolated from minced synovial tissue by dual immunofluorescent staining. J Rheumatol. 1990 Feb;17(2):142–148. [PubMed] [Google Scholar]
- Sander B., Andersson J., Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991 Feb;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Silverman E. D., Isacovics B., Petsche D., Laxer R. M. Synovial fluid cells in juvenile arthritis: evidence of selective T cell migration to inflamed tissue. Clin Exp Immunol. 1993 Jan;91(1):90–95. doi: 10.1111/j.1365-2249.1993.tb03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staite N. D., Richard K. A., Aspar D. G., Franz K. A., Galinet L. A., Dunn C. J. Induction of an acute erosive monarticular arthritis in mice by interleukin-1 and methylated bovine serum albumin. Arthritis Rheum. 1990 Feb;33(2):253–260. doi: 10.1002/art.1780330215. [DOI] [PubMed] [Google Scholar]
- Tetta C., Camussi G., Modena V., Di Vittorio C., Baglioni C. Tumour necrosis factor in serum and synovial fluid of patients with active and severe rheumatoid arthritis. Ann Rheum Dis. 1990 Sep;49(9):665–667. doi: 10.1136/ard.49.9.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. L., Beverley P. C. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990 Mar;69(3):460–467. [PMC free article] [PubMed] [Google Scholar]
- Westacott C. I., Whicher J. T., Barnes I. C., Thompson D., Swan A. J., Dieppe P. A. Synovial fluid concentration of five different cytokines in rheumatic diseases. Ann Rheum Dis. 1990 Sep;49(9):676–681. doi: 10.1136/ard.49.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Benedetti F., Massa M., Robbioni P., Ravelli A., Burgio G. R., Martini A. Correlation of serum interleukin-6 levels with joint involvement and thrombocytosis in systemic juvenile rheumatoid arthritis. Arthritis Rheum. 1991 Sep;34(9):1158–1163. doi: 10.1002/art.1780340912. [DOI] [PubMed] [Google Scholar]