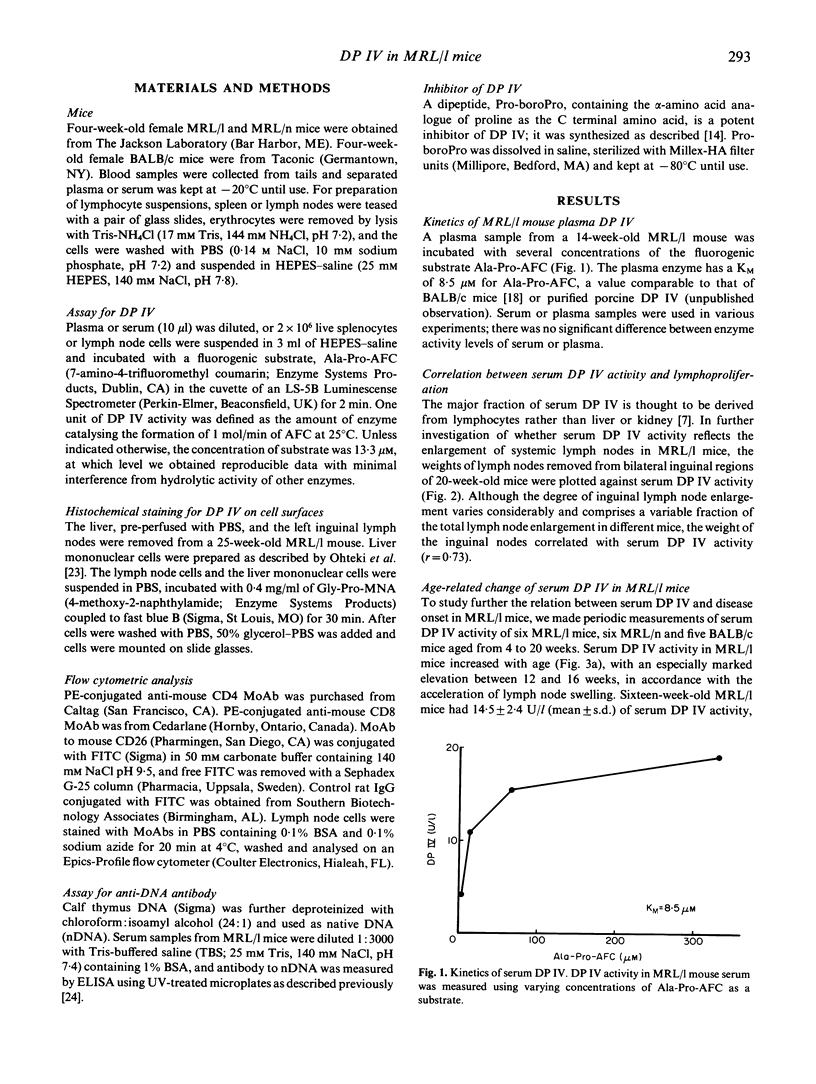
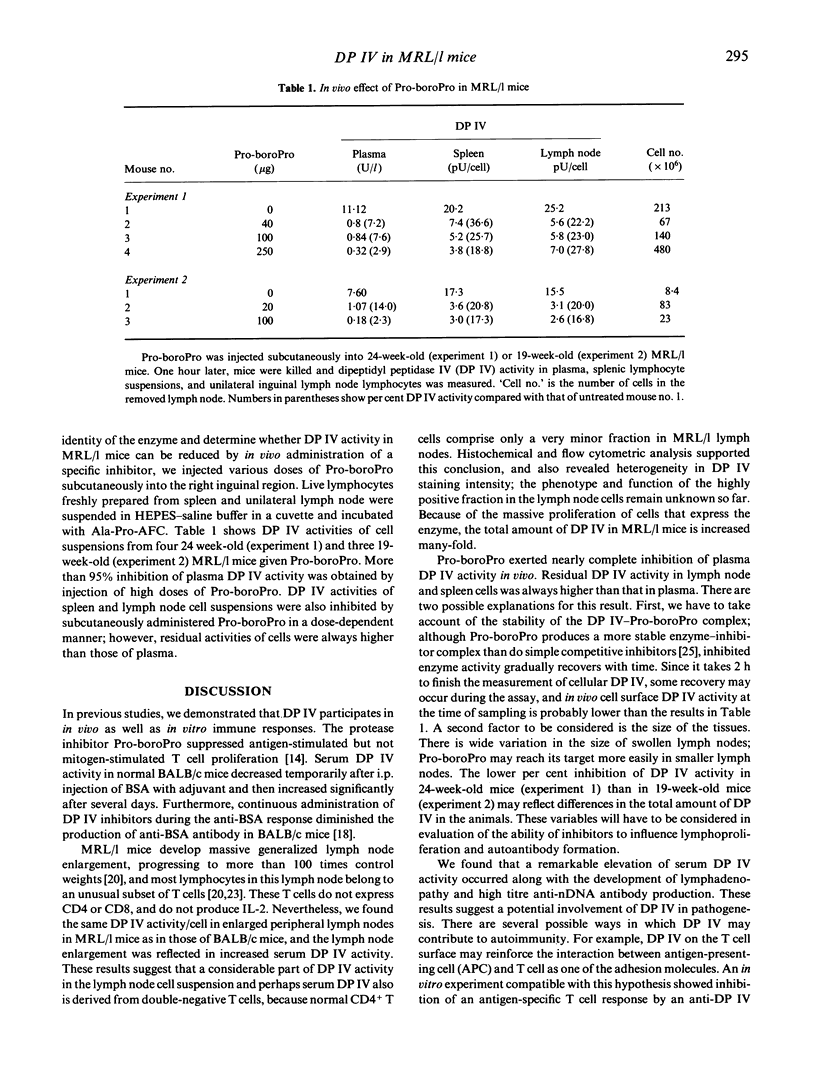
Abstract
DP IV (CD26), a serine protease expressed on activated T cells, participates in immune responses in vivo as well as in vitro. We measured cell surface and serum DP IV in mice of the autoimmune MRL/Mp-lpr/lpr (MRL/l) strain, which is characterized by massive T cell proliferation and production of anti-nuclear autoantibodies. The mass of inguinal lymph nodes correlated with serum DP IV activity. Furthermore, serum DP IV activity increased markedly in parallel with the acceleration of lymph node swelling and anti-nDNA antibody production. Serum DP IV activity in 16-week-old MRL/l mice reached levels up to three higher than those in age-matched MRL/Mp- +/+ mice or BALB/c mice. Immunohistochemical staining and flow cytometric analysis identified DV IV on surfaces of lymphocytes from the enlarged lymph nodes of MRL/l mice. Subcutaneous injection of the mechanism-based inhibitor, Pro-boroPro, reduced protease activity in serum and cell suspensions prepared from spleen and lymph nodes, confirming the identity of the enzyme as DP IV. These results indicate that the massively accumulating lymphocytes of MRL/l mice have a property characteristic of activated T cells, although they express little surface CD4 or CD8 and do not produce IL-2. DP IV may participate in the role these cells play in the pathogenesis of MRL/l autoimmune disease.
Full text
PDF


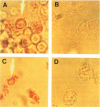
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dang N. H., Torimoto Y., Deusch K., Schlossman S. F., Morimoto C. Comitogenic effect of solid-phase immobilized anti-1F7 on human CD4 T cell activation via CD3 and CD2 pathways. J Immunol. 1990 Jun 1;144(11):4092–4100. [PubMed] [Google Scholar]
- Dang N. H., Torimoto Y., Sugita K., Daley J. F., Schow P., Prado C., Schlossman S. F., Morimoto C. Cell surface modulation of CD26 by anti-1F7 monoclonal antibody. Analysis of surface expression and human T cell activation. J Immunol. 1990 Dec 15;145(12):3963–3971. [PubMed] [Google Scholar]
- Flentke G. R., Munoz E., Huber B. T., Plaut A. G., Kettner C. A., Bachovchin W. W. Inhibition of dipeptidyl aminopeptidase IV (DP-IV) by Xaa-boroPro dipeptides and use of these inhibitors to examine the role of DP-IV in T-cell function. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1556–1559. doi: 10.1073/pnas.88.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrell M. D., Wickson J., McCaughan G. W. Expression of the rat CD26 antigen (dipeptidyl peptidase IV) on subpopulations of rat lymphocytes. Cell Immunol. 1991 Apr 15;134(1):205–215. doi: 10.1016/0008-8749(91)90343-a. [DOI] [PubMed] [Google Scholar]
- Gruber M., Scholz W., Flad H. D. Influence of human T lymphocytes identified by antibodies to dipeptidyl peptidase IV on differentiation of human B lymphocytes stimulated with Staphylococcus aureus Cowan I and pokeweed mitogen. Cell Immunol. 1988 May;113(2):423–434. doi: 10.1016/0008-8749(88)90039-1. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Fox D. A., Benjamin D., Weiner H. L. Antigen reactive memory T cells are defined by Ta1. J Immunol. 1986 Jul 15;137(2):414–418. [PubMed] [Google Scholar]
- Hanski C., Huhle T., Gossrau R., Reutter W. Direct evidence for the binding of rat liver DPP IV to collagen in vitro. Exp Cell Res. 1988 Sep;178(1):64–72. doi: 10.1016/0014-4827(88)90378-3. [DOI] [PubMed] [Google Scholar]
- Hegen M., Niedobitek G., Klein C. E., Stein H., Fleischer B. The T cell triggering molecule Tp103 is associated with dipeptidyl aminopeptidase IV activity. J Immunol. 1990 Apr 15;144(8):2908–2914. [PubMed] [Google Scholar]
- Hino M., Nagatsu T., Kakumu S., Okuyama S., Yoshii Y., Nagatsu I. Glycylprolyl beta-naphthylamidase activity in human serum. Clin Chim Acta. 1975 Jul 9;62(1):5–11. doi: 10.1016/0009-8981(75)90273-9. [DOI] [PubMed] [Google Scholar]
- Kameoka J., Tanaka T., Nojima Y., Schlossman S. F., Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993 Jul 23;261(5120):466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- Kettner C. A., Bone R., Agard D. A., Bachovchin W. W. Kinetic properties of the binding of alpha-lytic protease to peptide boronic acids. Biochemistry. 1988 Oct 4;27(20):7682–7688. doi: 10.1021/bi00420a017. [DOI] [PubMed] [Google Scholar]
- Kubota T., Flentke G. R., Bachovchin W. W., Stollar B. D. Involvement of dipeptidyl peptidase IV in an in vivo immune response. Clin Exp Immunol. 1992 Aug;89(2):192–197. doi: 10.1111/j.1365-2249.1992.tb06931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama J., Berman J. S., Cruikshank W. W., Morimoto C., Center D. M. Evidence for recent as well as long term activation of T cells migrating through endothelial cell monolayers in vitro. J Immunol. 1992 Mar 1;148(5):1367–1374. [PubMed] [Google Scholar]
- Mattern T., Scholz W., Feller A. C., Flad H. D., Ulmer A. J. Expression of CD26 (dipeptidyl peptidase IV) on resting and activated human T-lymphocytes. Scand J Immunol. 1991 Jun;33(6):737–748. doi: 10.1111/j.1365-3083.1991.tb02548.x. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Torimoto Y., Levinson G., Rudd C. E., Schrieber M., Dang N. H., Letvin N. L., Schlossman S. F. 1F7, a novel cell surface molecule, involved in helper function of CD4 cells. J Immunol. 1989 Dec 1;143(11):3430–3439. [PubMed] [Google Scholar]
- Morse H. C., 3rd, Davidson W. F., Yetter R. A., Murphy E. D., Roths J. B., Coffman R. L. Abnormalities induced by the mutant gene Ipr: expansion of a unique lymphocyte subset. J Immunol. 1982 Dec;129(6):2612–2615. [PubMed] [Google Scholar]
- Mountz J. D., Huppi K. E., Seldin M. F., Mushinski J. F., Steinberg A. D. T cell receptor gene expression in autoimmune mice. J Immunol. 1986 Aug 1;137(3):1029–1036. [PubMed] [Google Scholar]
- Ohteki T., Seki S., Abo T., Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4-8- double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990 Jul 1;172(1):7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plana M., Viñas O., De la Calle-Martin O., Lozano F., Inglés-Esteve J., Romero M., Alberola-Ila J., Yagüe J., Vilella R., Vives J. Induction of interleukin 2 (IL 2) and interferon-gamma and enhancement of IL 2 receptor expression by a CD26 monoclonal antibody. Eur J Immunol. 1991 Apr;21(4):1085–1088. doi: 10.1002/eji.1830210436. [DOI] [PubMed] [Google Scholar]
- Prud'Homme G. J., Park C. L., Fieser T. M., Kofler R., Dixon F. J., Theofilopoulos A. N. Identification of a B cell differentiation factor(s) spontaneously produced by proliferating T cells in murine lupus strains of the lpr/lpr genotype. J Exp Med. 1983 Feb 1;157(2):730–742. doi: 10.1084/jem.157.2.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz W., Mentlein R., Heymann E., Feller A. C., Ulmer A. J., Flad H. D. Interleukin 2 production by human T lymphocytes identified by antibodies to dipeptidyl peptidase IV. Cell Immunol. 1985 Jun;93(1):199–211. doi: 10.1016/0008-8749(85)90400-9. [DOI] [PubMed] [Google Scholar]
- Schön E., Jahn S., Kiessig S. T., Demuth H. U., Neubert K., Barth A., Von Baehr R., Ansorge S. The role of dipeptidyl peptidase IV in human T lymphocyte activation. Inhibitors and antibodies against dipeptidyl peptidase IV suppress lymphocyte proliferation and immunoglobulin synthesis in vitro. Eur J Immunol. 1987 Dec;17(12):1821–1826. doi: 10.1002/eji.1830171222. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Vivier I., Marguet D., Naquet P., Bonicel J., Black D., Li C. X., Bernard A. M., Gorvel J. P., Pierres M. Evidence that thymocyte-activating molecule is mouse CD26 (dipeptidyl peptidase IV). J Immunol. 1991 Jul 15;147(2):447–454. [PubMed] [Google Scholar]
- Vivier I., Marguet D., Naquet P., Bonicel J., Black D., Li C. X., Bernard A. M., Gorvel J. P., Pierres M. Evidence that thymocyte-activating molecule is mouse CD26 (dipeptidyl peptidase IV). J Immunol. 1991 Jul 15;147(2):447–454. [PubMed] [Google Scholar]
- Walter R., Simmons W. H., Yoshimoto T. Proline specific endo- and exopeptidases. Mol Cell Biochem. 1980 Apr 18;30(2):111–127. doi: 10.1007/BF00227927. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Zouali M., Stollar B. D. A rapid ELISA for measurement of antibodies to nucleic acid antigens using UV-treated polystyrene microplates. J Immunol Methods. 1986 Jun 10;90(1):105–110. doi: 10.1016/0022-1759(86)90390-x. [DOI] [PubMed] [Google Scholar]