Abstract
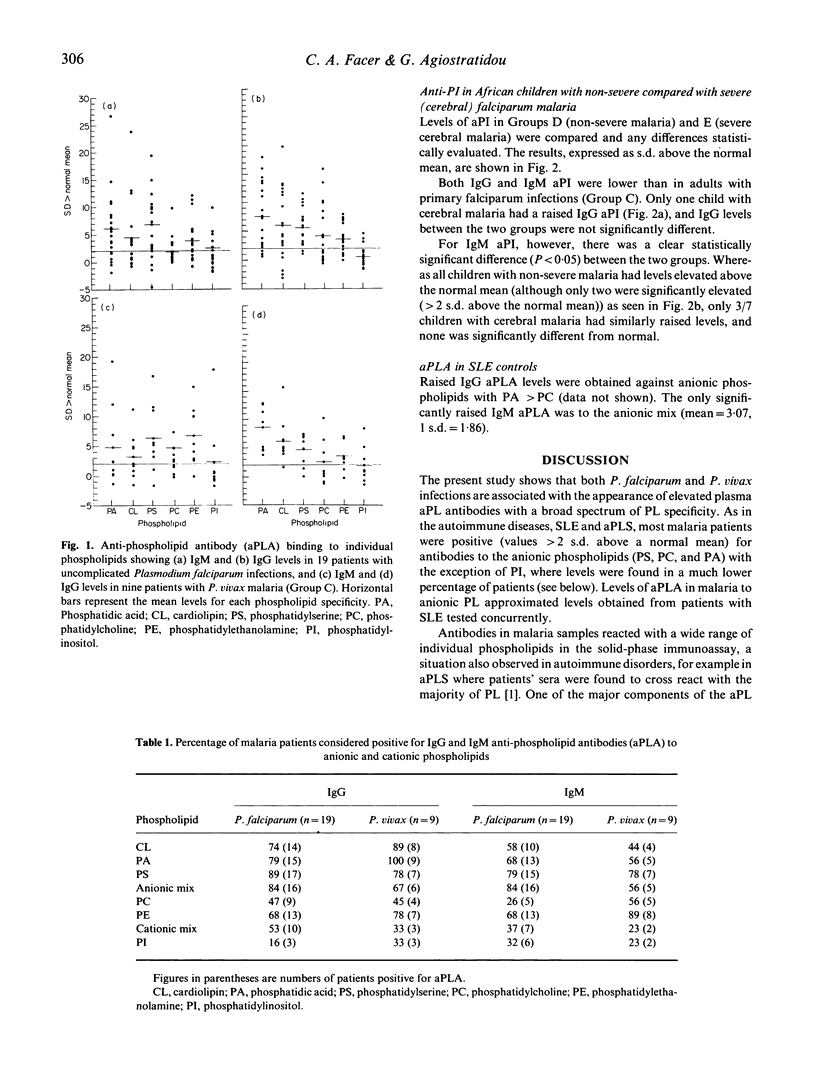
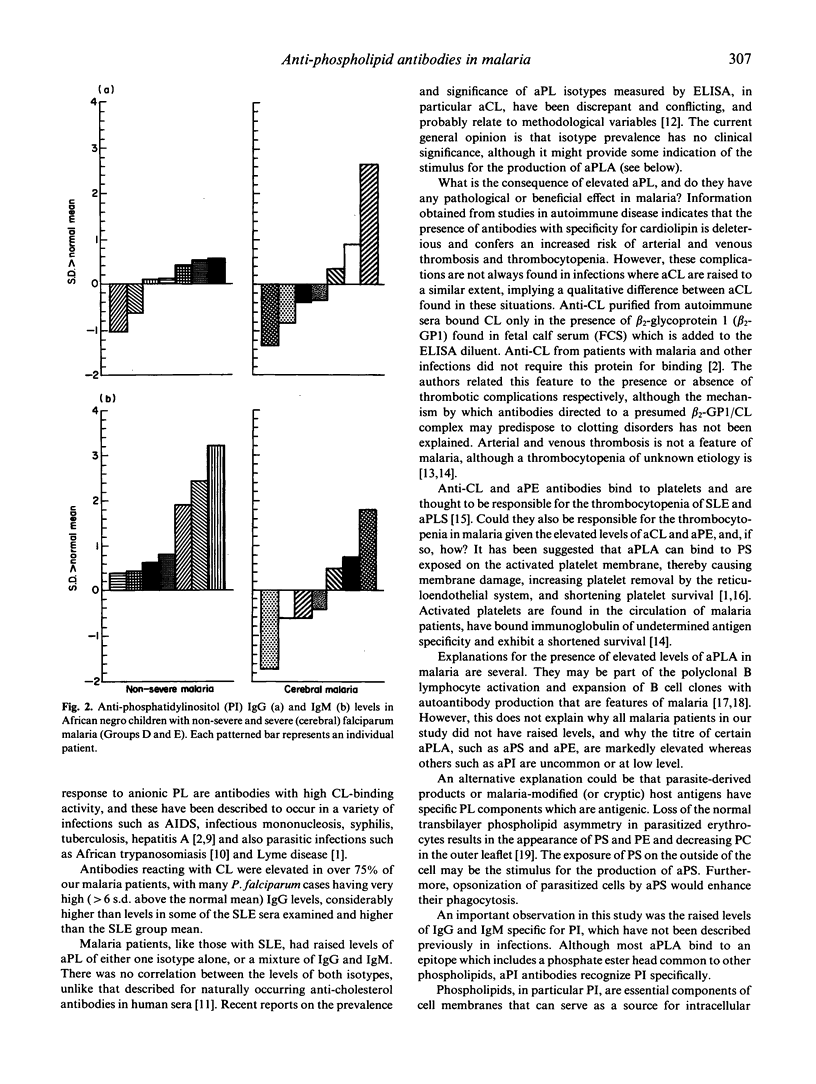
The majority (75%) of adult patients with uncomplicated Plasmodium falciparum and P. vivax malaria are positive for anti-phospholipid antibodies (aPLA) as demonstrated by ELISA using a panel of anionic and cationic phospholipids. The highest IgG and IgM binding was to the anionic phospholipids, phosphatidylserine (PS), phosphatidic acid (PA) and cardiolipin (CL), but excluding phosphatidylinositol (PI) to which only low antibody levels were found. Comparison of the mean IgG and IgM aPLA showed a trend for anti-PA > CL > PS > PC > PE > PI. Anti-PI levels were compared in two groups of African children, one group with non-severe and the other with severe (cerebral) falciparum malaria. Children with cerebral disease had significantly lower IgM anti-PI. The results are discussed with the view that serum-derived aPLA may have a role in 'anti-disease' immune responses. Their possible role in the opsonization and phagocytosis of parasitized erythrocytes and in thrombocytopenia is also considered.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adu D., Williams D. G., Quakyi I. A., Voller A., Anim-Addo Y., Bruce-Tagoe A. A., Johnson G. D., Holborow E. J. Anti-ssDNA and antinuclear antibodies in human malaria. Clin Exp Immunol. 1982 Aug;49(2):310–316. [PMC free article] [PubMed] [Google Scholar]
- Arvieux J., Roussel B., Ponard D., Colomb M. G. Reactivity patterns of anti-phospholipid antibodies in systemic lupus erythematosus sera in relation to erythrocyte binding and complement activation. Clin Exp Immunol. 1991 Jun;84(3):466–471. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Bootsma H. J., Mason R. C., Skalko N., Gregoriadis G., Playfair J. H. Antibodies against phosphatidylinositol and inositol monophosphate specifically inhibit tumour necrosis factor induction by malaria exoantigens. Immunology. 1992 May;76(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Playfair J. H. Soluble malarial antigens are toxic and induce the production of tumour necrosis factor in vivo. Immunology. 1989 Apr;66(4):600–605. [PMC free article] [PubMed] [Google Scholar]
- Bate C. A., Taverne J., Román E., Moreno C., Playfair J. H. Tumour necrosis factor induction by malaria exoantigens depends upon phospholipid. Immunology. 1992 Jan;75(1):129–135. [PMC free article] [PubMed] [Google Scholar]
- Facer C. A., Bray R. S., Brown J. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. I. Incidence and class specificity. Clin Exp Immunol. 1979 Jan;35(1):119–127. [PMC free article] [PubMed] [Google Scholar]
- Facer C. A. Direct Coombs antiglobulin reactions in Gambian children with Plasmodium falciparum malaria. II. Specificity of erythrocyte-bound IgG. Clin Exp Immunol. 1980 Feb;39(2):279–288. [PMC free article] [PubMed] [Google Scholar]
- Facer C. A., Jenkins G. C. Abnormal features of peripheral blood films from Gambian children with malaria. Ann Trop Paediatr. 1989 Jun;9(2):107–110. doi: 10.1080/02724936.1989.11748608. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Hunt J. E., McNeil H. P., Morgan G. J., Crameri R. M., Krilis S. A. A phospholipid-beta 2-glycoprotein I complex is an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus. 1992 Feb;1(2):75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- Kataaha P. K., Facer C. A., Mortazavi-Milani S. M., Stierle H., Holborow E. J. Stimulation of autoantibody production in normal blood lymphocytes by malaria culture supernatants. Parasite Immunol. 1984 Sep;6(5):481–492. doi: 10.1111/j.1365-3024.1984.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Kelton J. G., Keystone J., Moore J., Denomme G., Tozman E., Glynn M., Neame P. B., Gauldie J., Jensen J. Immune-mediated thrombocytopenia of malaria. J Clin Invest. 1983 Apr;71(4):832–836. doi: 10.1172/JCI110836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Wang C. T. Activation of human platelets by the rabbit anticardiolipin antibodies. Blood. 1992 Dec 15;80(12):3135–3143. [PubMed] [Google Scholar]
- Loizou S., Cofiner C., Weetman A. P., Walport M. J. Immunoglobulin class and IgG subclass distribution of anticardiolipin antibodies in patients with systemic lupus erythematosus and associated disorders. Clin Exp Immunol. 1992 Dec;90(3):434–439. doi: 10.1111/j.1365-2249.1992.tb05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackworth-Young C. Antiphospholipid antibodies: more than just a disease marker? Immunol Today. 1990 Feb;11(2):60–65. doi: 10.1016/0167-5699(90)90020-a. [DOI] [PubMed] [Google Scholar]
- Maguire P. A., Prudhomme J., Sherman I. W. Alterations in erythrocyte membrane phospholipid organization due to the intracellular growth of the human malaria parasite, Plasmodium falciparum. Parasitology. 1991 Apr;102(Pt 2):179–186. doi: 10.1017/s0031182000062466. [DOI] [PubMed] [Google Scholar]
- McNeil H. P., Chesterman C. N., Krilis S. A. Immunology and clinical importance of antiphospholipid antibodies. Adv Immunol. 1991;49:193–280. doi: 10.1016/s0065-2776(08)60777-4. [DOI] [PubMed] [Google Scholar]
- Molyneux M. E., Taylor T. E., Wirima J. J., Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med. 1989 May;71(265):441–459. [PubMed] [Google Scholar]
- Playfair J. H., Taverne J., Bate C. A., de Souza J. B. The malaria vaccine: anti-parasite or anti-disease? Immunol Today. 1990 Jan;11(1):25–27. doi: 10.1016/0167-5699(90)90007-v. [DOI] [PubMed] [Google Scholar]
- Richards R. L., Aronson J., Schoenbechler M., Diggs C. L., Alving C. R. Antibodies reactive with liposomal phospholipids are produced during experimental Trypanosoma rhodesiense infections in rabbits. J Immunol. 1983 Mar;130(3):1390–1394. [PubMed] [Google Scholar]
- Schofield L., Hackett F. Signal transduction in host cells by a glycosylphosphatidylinositol toxin of malaria parasites. J Exp Med. 1993 Jan 1;177(1):145–153. doi: 10.1084/jem.177.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K., Bate C. A., Carr R. E., Butcher G. A., Taverne J., Playfair J. H. Phospholipid-containing toxic malaria antigens induce hypoglycaemia. Clin Exp Immunol. 1992 Oct;90(1):1–5. doi: 10.1111/j.1365-2249.1992.tb05822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaarala O., Palosuo T., Kleemola M., Aho K. Anticardiolipin response in acute infections. Clin Immunol Immunopathol. 1986 Oct;41(1):8–15. doi: 10.1016/0090-1229(86)90046-2. [DOI] [PubMed] [Google Scholar]


