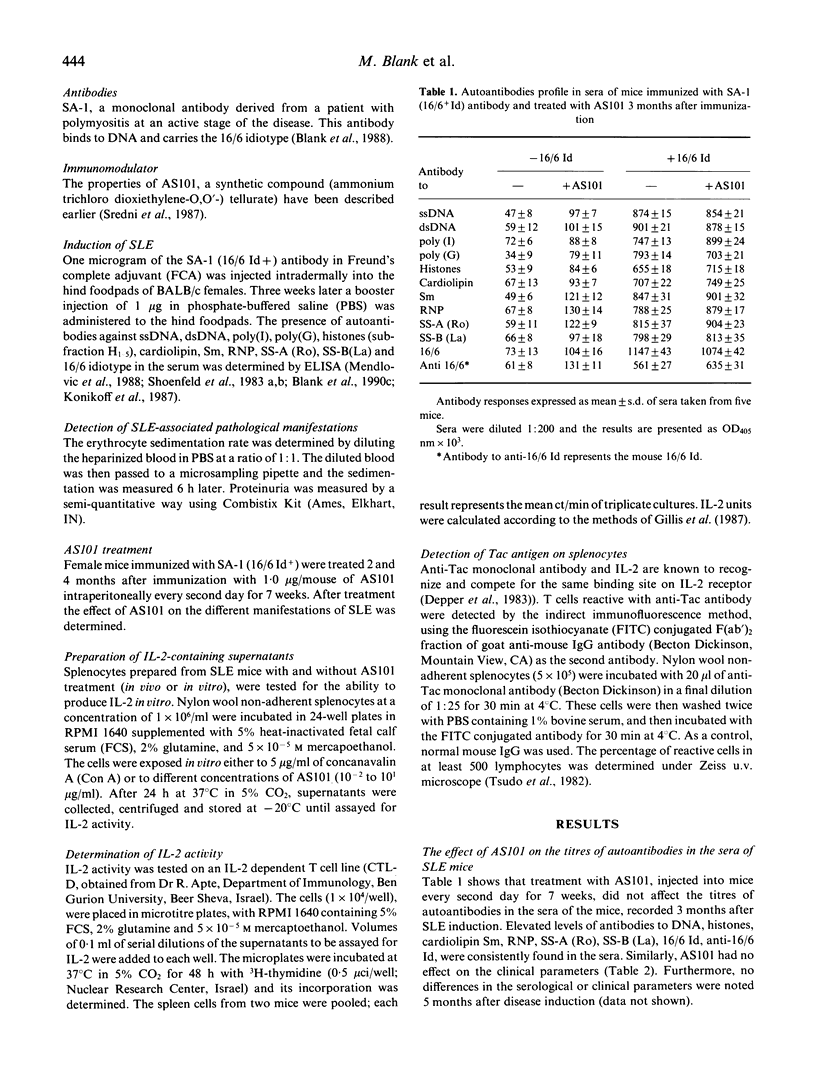
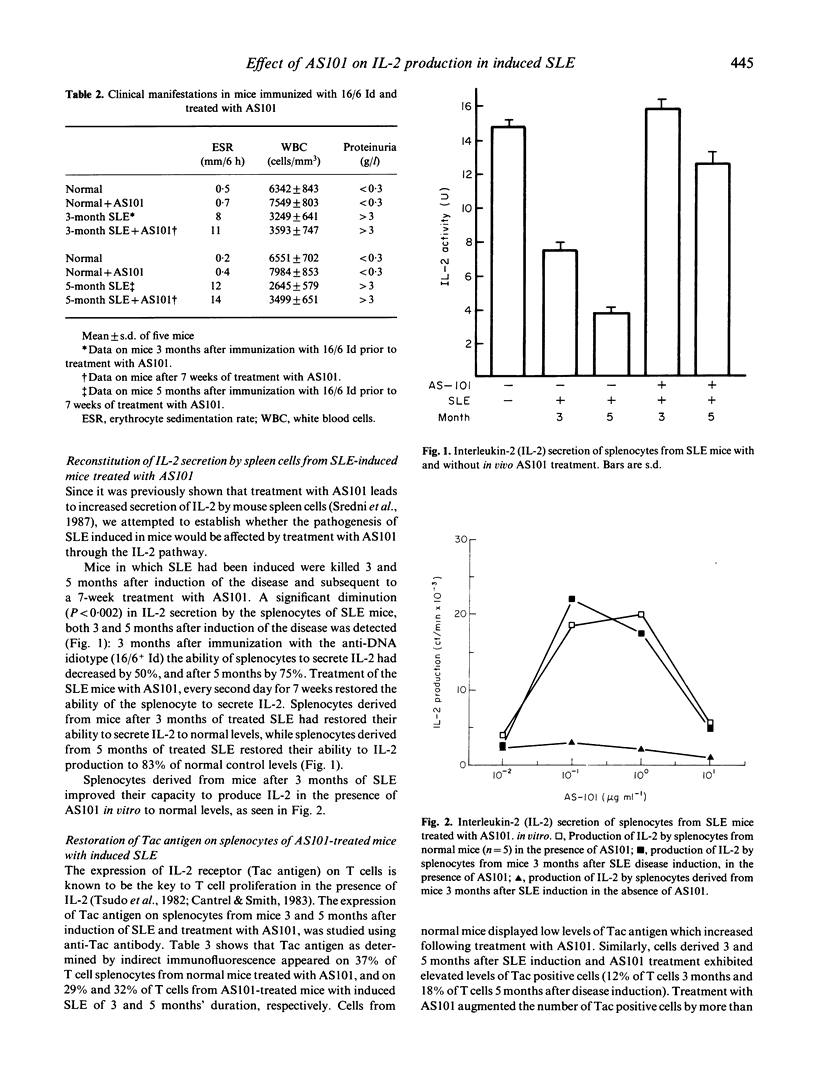
Abstract
The role of the synthetic immunomodulator AS101 on the production of interleukin-2 (IL-2) by spleen cells of mice with SLE was investigated. BALB/c female mice, in which SLE was induced by immunization with the pathogenic idiotype of anti-DNA antibody 16/6 Id were treated with AS101 for 7 weeks 2 and 4 months after induction of the disease. The ability of the splenocytes of the mice with SLE to produce IL-2 was restored after administration of AS101. This effect was particularly impressive when the 7-week AS101 treatment was initiated 4 months after immunization. Despite its beneficial effect on IL-2 production, AS101 exerted no influence on the titres of autoantibodies in the sera of the mice. It also had no effect on clinical parameters of SLE, such as the increased sedimentation rate, proteinuria and low white blood cell counts. Our data indicate that defective IL-2 production in SLE is probably secondary to other disease processes and is not necessarily associated with the production of autoantibodies in this disorder.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcocer-Varela J., Alarcón-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982 Jun;69(6):1388–1392. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcocer-Varela J., Laffón A., Alarcón-Segovia D. Differences in the production of and/or the response to interleukin-2 by T lymphocytes from patients with the various connective tissue diseases. Rheumatol Int. 1984;4(1):39–44. doi: 10.1007/BF00683884. [DOI] [PubMed] [Google Scholar]
- Altman A., Theofilopoulos A. N., Weiner R., Katz D. H., Dixon F. J. Analysis of T cell function in autoimmune murine strains. Defects in production and responsiveness to interleukin 2. J Exp Med. 1981 Sep 1;154(3):791–808. doi: 10.1084/jem.154.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy A. S., Calder V. L., Feldmann M., Davison A. N. The distribution of interleukin-2 receptor bearing lymphocytes in multiple sclerosis: evidence for a key role of activated lymphocytes. Clin Exp Immunol. 1985 Aug;61(2):248–256. [PMC free article] [PubMed] [Google Scholar]
- Blaese R. M., Grayson J., Steinberg A. D. Increased immunoglobulin-secreting cells in the blood of patients with active systemic lupus erythematosus. Am J Med. 1980 Sep;69(3):345–350. doi: 10.1016/0002-9343(80)90003-0. [DOI] [PubMed] [Google Scholar]
- Blank M., Krup M., Mendlovic S., Fricke H., Mozes E., Talal N., Coates A. R., Shoenfeld Y. The importance of the pathogenic 16/6 idiotype in the induction of SLE in naive mice. Scand J Immunol. 1990 Jan;31(1):45–52. doi: 10.1111/j.1365-3083.1990.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Bocchieri M. H., Knittweis L., Seaton D. S. Cytokine production by NZB, C58, and NZB X C58 recombinant inbred mice. Cell Immunol. 1984 Oct 15;88(2):453–463. doi: 10.1016/0008-8749(84)90177-1. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983 Dec 1;158(6):1895–1911. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathely G., Amor B., Fournier C. Defective IL2 production in active rheumatoid arthritis. Regulation by radiosensitive suppressor cells. Clin Rheumatol. 1986 Dec;5(4):482–492. [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Coordinate expression of macrophage procoagulant and fibrinolytic activity in vitro and in vivo. J Immunol. 1983 Jan;130(1):261–266. [PubMed] [Google Scholar]
- Decker J. L., Steinberg A. D., Reinertsen J. L., Plotz P. H., Balow J. E., Klippel J. H. NIH conference. Systemic lupus erythematosus: evolving concepts. Ann Intern Med. 1979 Oct;91(4):587–604. doi: 10.7326/0003-4819-91-4-587. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Robb R. J., Waldmann T. A., Greene W. C. Blockade of the interleukin-2 receptor by anti-Tac antibody: inhibition of human lymphocyte activation. J Immunol. 1983 Aug;131(2):690–696. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goto M., Tanimoto K., Horiuchi Y. Natural cell mediated cytotoxicity in systemic lupus erythematosus: suppression by antilymphocyte antibody. Arthritis Rheum. 1980 Nov;23(11):1274–1281. doi: 10.1002/art.1780231108. [DOI] [PubMed] [Google Scholar]
- Horwitz D. A., Garrett M. A. Lymphocyte reactivity to mitogens in subjects with systemic lupus erythematosus, rheumatoid arthritis and scleroderma. Clin Exp Immunol. 1977 Jan;27(1):92–99. [PMC free article] [PubMed] [Google Scholar]
- Huang Y. P., Perrin L. H., Miescher P. A., Zubler R. H. Correlation of T and B cell activities in vitro and serum IL-2 levels in systemic lupus erythematosus. J Immunol. 1988 Aug 1;141(3):827–833. [PubMed] [Google Scholar]
- Katz P., Zaytoun A. M., Lee J. H., Jr, Panush R. S., Longley S. Abnormal natural killer cell activity in systemic lupus erythematosus: an intrinsic defect in the lytic event. J Immunol. 1982 Nov;129(5):1966–1971. [PubMed] [Google Scholar]
- Konikoff F., Isenberg D. A., Kooperman O., Kennedy R. C., Rauch J., Theodor E., Shoenfeld Y. Common lupus anti-DNA antibody idiotypes in chronic liver diseases. Clin Immunol Immunopathol. 1987 May;43(2):265–272. doi: 10.1016/0090-1229(87)90134-6. [DOI] [PubMed] [Google Scholar]
- Mendlovic S., Brocke S., Shoenfeld Y., Ben-Bassat M., Meshorer A., Bakimer R., Mozes E. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2260–2264. doi: 10.1073/pnas.85.7.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendlovic S., Fricke H., Shoenfeld Y., Mozes E. The role of anti-idiotypic antibodies in the induction of experimental systemic lupus erythematosus in mice. Eur J Immunol. 1989 Apr;19(4):729–734. doi: 10.1002/eji.1830190424. [DOI] [PubMed] [Google Scholar]
- Murakawa Y., Takada S., Ueda Y., Suzuki N., Hoshino T., Sakane T. Characterization of T lymphocyte subpopulations responsible for deficient interleukin 2 activity in patients with systemic lupus erythematosus. J Immunol. 1985 Jan;134(1):187–195. [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A., Rauch J., Madaio M. P., Stollar B. D., Schwartz R. S. Idiotypic cross-reactions of monoclonal human lupus autoantibodies. J Exp Med. 1983 Sep 1;158(3):718–730. doi: 10.1084/jem.158.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoenfeld Y., Rauch J., Massicotte H., Datta S. K., André-Schwartz J., Stollar B. D., Schwartz R. S. Polyspecificity of monoclonal lupus autoantibodies produced by human-human hybridomas. N Engl J Med. 1983 Feb 24;308(8):414–420. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Teplizki H. A., Mendlovic S., Blank M., Mozes E., Isenberg D. A. The role of the human anti-DNA idiotype 16/6 in autoimmunity. Clin Immunol Immunopathol. 1989 Jun;51(3):313–325. doi: 10.1016/0090-1229(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Sredni B., Caspi R. R., Klein A., Kalechman Y., Danziger Y., Ben Ya'akov M., Tamari T., Shalit F., Albeck M. A new immunomodulating compound (AS-101) with potential therapeutic application. Nature. 1987 Nov 12;330(6144):173–176. doi: 10.1038/330173a0. [DOI] [PubMed] [Google Scholar]
- Sredni B., Caspi R. R., Lustig S., Klein A., Kalechman Y., Danziger Y., Ben Ya'akov M., Tamari T., Shalit F., Albeck M. The biological activity and immunotherapeutic properties of AS-101, a synthetic organotellurium compound. Nat Immun Cell Growth Regul. 1988;7(3):163–168. [PubMed] [Google Scholar]
- Tsudo M., Uchiyama T., Takatsuki K., Uchino H., Yodoi J. Modulation of Tac antigen on activated human T cells by anti-Tac monoclonal antibody. J Immunol. 1982 Aug;129(2):592–595. [PubMed] [Google Scholar]
- Wofsy D., Murphy E. D., Roths J. B., Dauphinée M. J., Kipper S. B., Talal N. Deficient interleukin 2 activity in MRL/Mp and C57BL/6J mice bearing the lpr gene. J Exp Med. 1981 Nov 1;154(5):1671–1680. doi: 10.1084/jem.154.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]


