Abstract
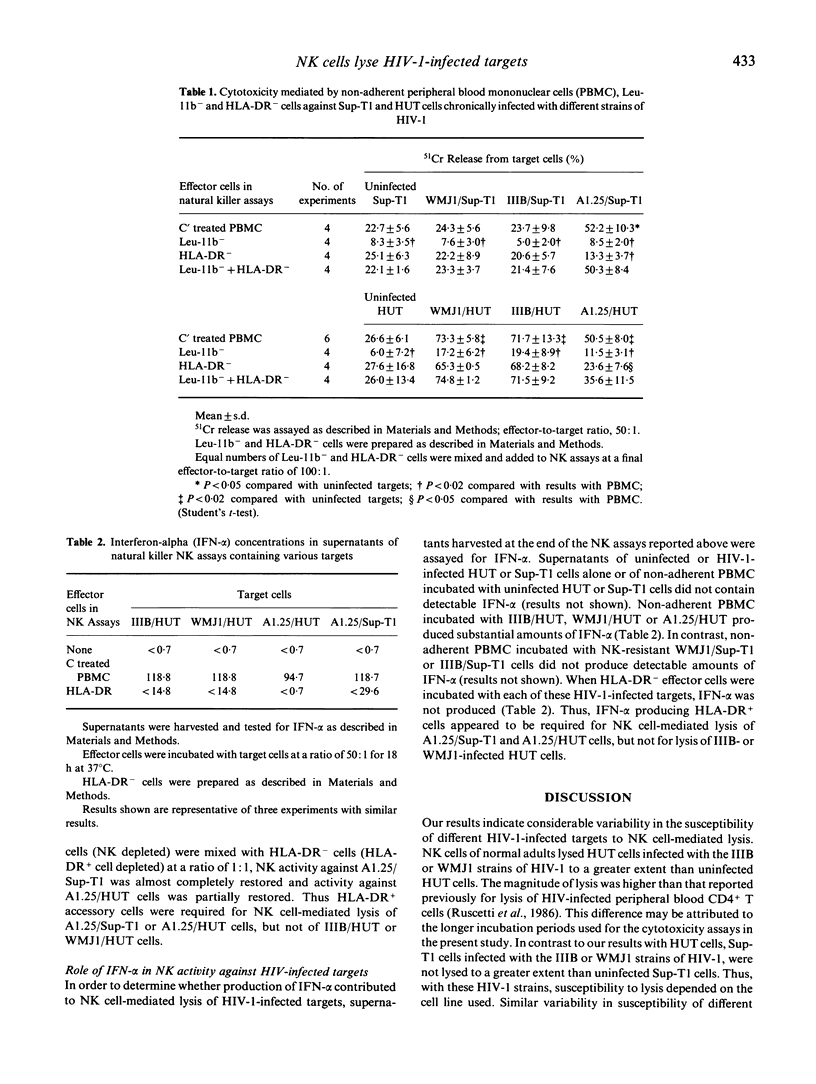
The susceptibility of HIV-1-infected CD4+ T cell lines to natural killer (NK) cell-mediated lysis was examined. Non-adherent peripheral blood mononuclear cells (PBMC) of healthy adults lysed HUT cells chronically infected with the IIIB or WMJ1 strains of HIV-1 to a significantly greater extent than uninfected HUT cells. In contrast, Sup-T1 cells chronically infected with these two strains of HIV-1 were not lysed to a greater extent than uninfected Sup-T1 cells. Clone A1.25-infected Sup-T1 (A1.25/Sup-T1), derived from IIIB-infected Sup-T1 cells (IIIB/Sup-T1), were susceptible to non-adherent PBMC-mediated lysis, as were A1.25-infected HUT cells (A1.25/HUT). When non-adherent PBMC were depleted of CD16 (Leu-11b)+ NK cells by treatment with anti-Leu-11b plus C, lysis of HIV-1-infected HUT or Sup-T1 cells was reduced to low levels, indicating that the lysis was mediated by NK cells. Expression of HIV antigens on these target cells did not correlate with their susceptibility to NK cell-mediated lysis. Depletion of interferon-alpha (IFN-alpha) producing HLA-DR+ cells from non-adherent PBMC had no effect on the magnitude of NK cell-mediated lysis of IIIB or WMJ1-infected HUT cells. In contrast, lysis of A1.25/Sup-T1 or A1.25/HUT cells required the presence of HLA-DR+ cells. IFN-alpha production appeared to be required for NK cell-mediated lysis of A1.25/Sup-T1 or A1.25/HUT cells, while lysis of HUT cells infected with the WMJ1 or IIIB strains of HIV-1 was IFN-alpha independent. These results indicate considerable variability in the susceptibility of different HIV-1 infected T cell lines to NK cell-mediated lysis and suggest the existence of alternative mechanisms of activation of NK cells for lysis of HIV-1-infected T cell lines.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbeit R. D., Leary P. L., Levin M. J. Gamma interferon production by combinations of human peripheral blood lymphocytes, monocytes, and cultured macrophages. Infect Immun. 1982 Feb;35(2):383–390. doi: 10.1128/iai.35.2.383-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S., Oh S. H., Michelson S., Miller D. S., Virelizier J. L., Starr S. E. Natural killing of fibroblasts infected with low-passage clinical isolates of human cytomegalovirus. Clin Exp Immunol. 1988 Jul;73(1):11–16. [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S., Perussia B., Trinchieri G., Miller D. S., Starr S. E. Requirement for HLA-DR+ accessory cells in natural killing of cytomegalovirus-infected fibroblasts. J Exp Med. 1986 Jul 1;164(1):180–195. doi: 10.1084/jem.164.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. A., Mendelsohn M., Lopez C. Human natural killer cells limit replication of herpes simplex virus type 1 in vitro. J Immunol. 1985 Apr;134(4):2666–2672. [PubMed] [Google Scholar]
- Getchell J. P., Heath J. L., Hicks D. R., Sporborg C., McGrath C. R., Kalyanaraman V. S. Continuous production of a cytopathic human T-lymphotropic virus in a permissive neoplastic T-cell line. J Clin Microbiol. 1986 Apr;23(4):737–742. doi: 10.1128/jcm.23.4.737-742.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootenberg J. E., Ruscetti F. W., Mier J. W., Gazdar A., Gallo R. C. Human cutaneous T cell lymphoma and leukemia cell lines produce and respond to T cell growth factor. J Exp Med. 1981 Nov 1;154(5):1403–1418. doi: 10.1084/jem.154.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Bandu M. T., Brouty-boye D., Tovey M. Pronounced antiviral activity of human interferon on bovine and porcine cells. Nature. 1974 Oct 11;251(5475):543–545. doi: 10.1038/251543a0. [DOI] [PubMed] [Google Scholar]
- Herberman R. B. Natural killer (NK) cells and their possible roles in resistance against disease. Clin Immunol Rev. 1981;1(1):1–65. [PubMed] [Google Scholar]
- Hoxie J. A., Alpers J. D., Rackowski J. L., Huebner K., Haggarty B. S., Cedarbaum A. J., Reed J. C. Alterations in T4 (CD4) protein and mRNA synthesis in cells infected with HIV. Science. 1986 Nov 28;234(4780):1123–1127. doi: 10.1126/science.3095925. [DOI] [PubMed] [Google Scholar]
- Hoxie J. A., Haggarty B. S., Rackowski J. L., Pillsbury N., Levy J. A. Persistent noncytopathic infection of normal human T lymphocytes with AIDS-associated retrovirus. Science. 1985 Sep 27;229(4720):1400–1402. doi: 10.1126/science.2994222. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Burioni R., Ar-Rushdi A., Zmijewski C., Croce C. M. Expression of members of immunoglobulin gene family in somatic cell hybrids between human B and T cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4969–4973. doi: 10.1073/pnas.84.14.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai K., Itoh K., Hinuma S., Tada M. Pretreatment of plastic Petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods. 1979;29(1):17–25. doi: 10.1016/0022-1759(79)90121-2. [DOI] [PubMed] [Google Scholar]
- Lyerly H. K., Matthews T. J., Langlois A. J., Bolognesi D. P., Weinhold K. J. Human T-cell lymphotropic virus IIIB glycoprotein (gp120) bound to CD4 determinants on normal lymphocytes and expressed by infected cells serves as target for immune attack. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4601–4605. doi: 10.1073/pnas.84.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Philpott K., Dalgleish A. G., Mellor A. L., Patterson S., Webster A. D., Edwards A. J., Maddon P. J. Infection of B lymphocytes by the human immunodeficiency virus and their susceptibility to cytotoxic cells. Eur J Immunol. 1988 Sep;18(9):1315–1321. doi: 10.1002/eji.1830180903. [DOI] [PubMed] [Google Scholar]
- Oh S. H., Bandyopadhyay S., Miller D. S., Starr S. E. Cooperation between CD16(Leu-11b)+ NK cells and HLA-DR+ cells in natural killing of herpesvirus-infected fibroblasts. J Immunol. 1987 Oct 15;139(8):2799–2802. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Jackson A., Warner N. L., Faust J., Rumpold H., Kraft D., Lanier L. L. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984 Jul;133(1):180–189. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Lebman D., Jankiewicz J., Lange B., Rovera G. Monoclonal antibodies that detect differentiation surface antigens on human myelomonocytic cells. Blood. 1982 Feb;59(2):382–392. [PubMed] [Google Scholar]
- Plata F., Autran B., Martins L. P., Wain-Hobson S., Raphaël M., Mayaud C., Denis M., Guillon J. M., Debré P. AIDS virus-specific cytotoxic T lymphocytes in lung disorders. Nature. 1987 Jul 23;328(6128):348–351. doi: 10.1038/328348a0. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Mikovits J. A., Kalyanaraman V. S., Overton R., Stevenson H., Stromberg K., Herberman R. B., Farrar W. L., Ortaldo J. R. Analysis of effector mechanisms against HTLV-I- and HTLV-III/LAV-infected lymphoid cells. J Immunol. 1986 May 15;136(10):3619–3624. [PubMed] [Google Scholar]
- Siliciano R. F., Lawton T., Knall C., Karr R. W., Berman P., Gregory T., Reinherz E. L. Analysis of host-virus interactions in AIDS with anti-gp120 T cell clones: effect of HIV sequence variation and a mechanism for CD4+ cell depletion. Cell. 1988 Aug 12;54(4):561–575. doi: 10.1016/0092-8674(88)90078-5. [DOI] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Welsh R. M. Regulation of virus infections by natural killer cells. A review. Nat Immun Cell Growth Regul. 1986;5(4):169–199. [PubMed] [Google Scholar]


