Abstract
We studied the immunologic correlates of disease activity and differences among subgroups of patients with idiopathic inflammatory myopathy by analysing phenotypic and activation marker expression on peripheral blood mononuclear cells (PBMC). Compared with controls, myositis patients with clinically active disease (n = 51) had significantly lower proportions of CD8+ cells and higher proportions of PBMC that expressed DR, CD3- DR, CD14- DR, interleukin-2 receptors, and the late T cell activation markers CD26 and TLiSA1. TLiSA1 expression, a marker for cytotoxic differentiation, correlated significantly with both clinical activity indices and serum levels of muscle-associated enzymes. In serial studies of seven patients, the proportion of PBMC expressing MHC class II antigen and late T cell activation markers decreased as myositis disease activity decreased, independent of type of therapy. Among the clinical subgroups, polymyositis (n = 21) and inclusion body myositis (n = 11) were virtually indistinguishable; dermatomyositis patients (n = 19) showed decreased proportions of CD3+DR+ and TLiSA1+ cells, and increased proportions of CD20+ and CD20+DR+ cells compared with the other two groups. Patients with autoantibodies to histidyl-tRNA synthetase (Jo-1 antigen, n = 11) had significantly lower proportions of CD3+ and CD4+ cells, lower CD4/CD8 ratios, and higher proportions of CD+ cells expressing CD20, compared with patients without anti-Jo-1 antibodies. These findings support the concept that activated lymphocytes, especially cells undergoing anamnestic responses and cytotoxic differentiation, are important in the pathogenesis of idiopathic myositis. Moreover, taken together with other studies, these data suggest that groups of patients segregated by clinical or autoantibody status have different mechanisms of systemic immune activation and immunopathology.
Full text
PDF


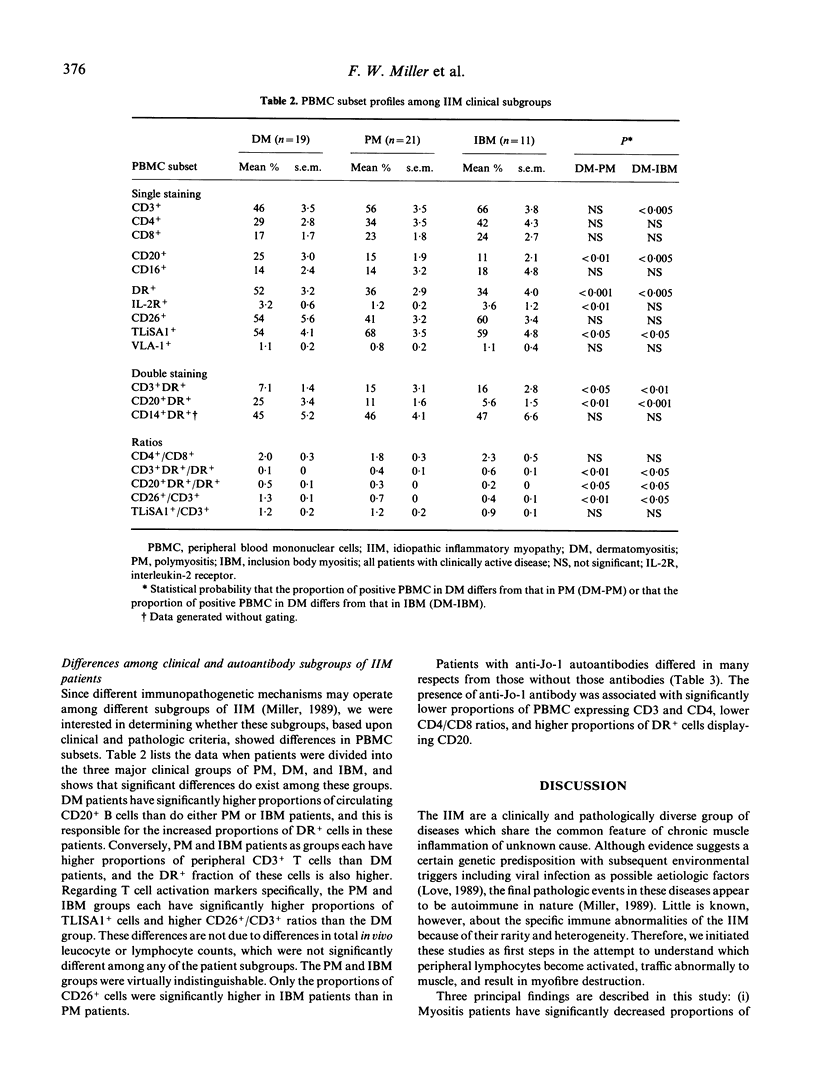
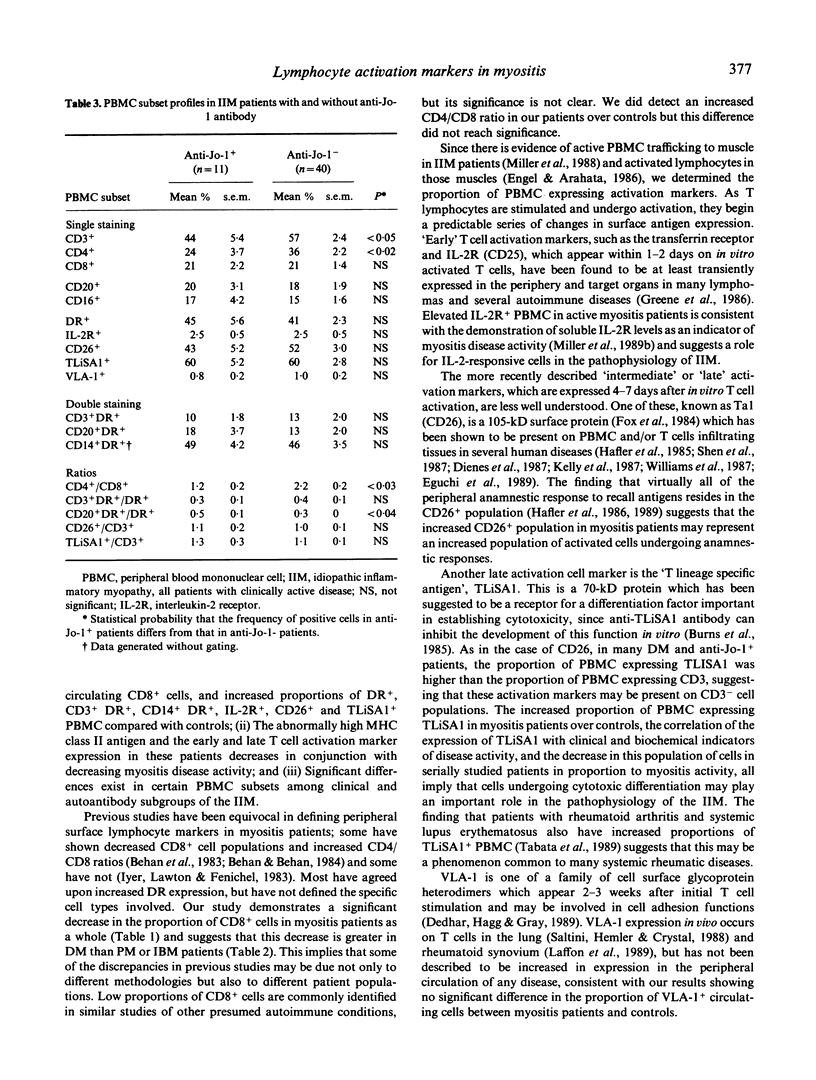


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behan W. M., Behan P. O. Disturbance of regulatory lymphocyte subsets in polymyositis. Ann Neurol. 1984 Jan;15(1):112–114. doi: 10.1002/ana.410150126. [DOI] [PubMed] [Google Scholar]
- Behan W. M., Behan P. O., Micklem H. S., Durward W. F. Abnormalities of lymphocyte subsets in polymyositis. Br Med J (Clin Res Ed) 1983 Jul 16;287(6386):181–182. doi: 10.1136/bmj.287.6386.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas T., Miller F. W., Takagaki Y., Plotz P. H. An enzyme-linked immunosorbent assay for the detection and quantitation of anti-Jo-1 antibody in human serum. J Immunol Methods. 1987 Apr 16;98(2):243–248. doi: 10.1016/0022-1759(87)90011-1. [DOI] [PubMed] [Google Scholar]
- Bohan A., Peter J. B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975 Feb 13;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- Burns G. F., Triglia T., Werkmeister J. A., Begley C. G., Boyd A. W. TLiSA1, a human T lineage-specific activation antigen involved in the differentiation of cytotoxic T lymphocytes and anomalous killer cells from their precursors. J Exp Med. 1985 May 1;161(5):1063–1078. doi: 10.1084/jem.161.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cronin M. E., Plotz P. H., Miller F. W. Abnormalities of the immune system in the idiopathic inflammatory myopathies. In Vivo. 1988 Jan-Feb;2(1):25–29. [PubMed] [Google Scholar]
- Dedhar S., Haqq C., Gray V. Specific overproduction of very late antigen 1 integrin in two human neuroblastoma cell lines selected for resistance to detachment by an Arg-Gly-Asp-containing synthetic peptide. J Biol Chem. 1989 Mar 25;264(9):4832–4836. [PubMed] [Google Scholar]
- Dienes H. P., Hütteroth T., Hess G., Meuer S. C. Immunoelectron microscopic observations on the inflammatory infiltrates and HLA antigens in hepatitis B and non-A, non-B. Hepatology. 1987 Nov-Dec;7(6):1317–1325. doi: 10.1002/hep.1840070623. [DOI] [PubMed] [Google Scholar]
- Eguchi K., Ueki Y., Shimomura C., Otsubo T., Nakao H., Migita K., Kawakami A., Matsunaga M., Tezuka H., Ishikawa N. Increment in the Ta1+ cells in the peripheral blood and thyroid tissue of patients with Graves' disease. J Immunol. 1989 Jun 15;142(12):4233–4240. [PubMed] [Google Scholar]
- Engel A. G., Arahata K. Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986 Jul;17(7):704–721. doi: 10.1016/s0046-8177(86)80180-0. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Acuto O., Poole C., Palley L., Daley J. F., Schlossman S. F., Reinherz E. L. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984 Sep;133(3):1250–1256. [PubMed] [Google Scholar]
- Greene W. C., Leonard W. J., Depper J. M., Nelson D. L., Waldmann T. A. The human interleukin-2 receptor: normal and abnormal expression in T cells and in leukemias induced by the human T-lymphotropic retroviruses. Ann Intern Med. 1986 Oct;105(4):560–572. doi: 10.7326/0003-4819-105-4-560. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Chofflon M., Benjamin D., Dang N. H., Breitmeyer J. Mechanisms of immune memory. T cell activation and CD3 phosphorylation correlates with Ta1 (CDw26) expression. J Immunol. 1989 Apr 15;142(8):2590–2596. [PubMed] [Google Scholar]
- Hafler D. A., Fox D. A., Benjamin D., Weiner H. L. Antigen reactive memory T cells are defined by Ta1. J Immunol. 1986 Jul 15;137(2):414–418. [PubMed] [Google Scholar]
- Hafler D. A., Fox D. A., Manning M. E., Schlossman S. F., Reinherz E. L., Weiner H. L. In vivo activated T lymphocytes in the peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. N Engl J Med. 1985 May 30;312(22):1405–1411. doi: 10.1056/NEJM198505303122201. [DOI] [PubMed] [Google Scholar]
- Iyer V., Lawton A. R., Fenichel G. M. T-cell subsets in polymyositis. Ann Neurol. 1983 Apr;13(4):452–453. doi: 10.1002/ana.410130413. [DOI] [PubMed] [Google Scholar]
- Kalovidouris A. E., Pourmand R., Passo M. H., Plotkin Z. Proliferative response of peripheral blood mononuclear cells to autologous and allogeneic muscle in patients with polymyositis/dermatomyositis. Arthritis Rheum. 1989 Apr;32(4):446–453. doi: 10.1002/anr.1780320414. [DOI] [PubMed] [Google Scholar]
- Kelly J., O'Farrelly C., O'Mahony C., Weir D. G., Feighery C. Immunoperoxidase demonstration of the cellular composition of the normal and coeliac small bowel. Clin Exp Immunol. 1987 Apr;68(1):177–188. [PMC free article] [PubMed] [Google Scholar]
- Kissel J. T., Mendell J. R., Rammohan K. W. Microvascular deposition of complement membrane attack complex in dermatomyositis. N Engl J Med. 1986 Feb 6;314(6):329–334. doi: 10.1056/NEJM198602063140601. [DOI] [PubMed] [Google Scholar]
- Laffon A., Sánchez-Madrid F., Ortíz de Landázuri M., Jiménez Cuesta A., Ariza A., Ossorio C., Sabando P. Very late activation antigen on synovial fluid T cells from patients with rheumatoid arthritis and other rheumatic diseases. Arthritis Rheum. 1989 Apr;32(4):386–392. doi: 10.1002/anr.1780320405. [DOI] [PubMed] [Google Scholar]
- Miller M. H., Powell J. I., Sharrow S. O., Schultz A. R. Rapid data collection, analysis, and graphics for flow microfluorometry instrumentation. Rev Sci Instrum. 1978 Aug;49(8):1137–1142. doi: 10.1063/1.1135535. [DOI] [PubMed] [Google Scholar]
- Saltini C., Hemler M. E., Crystal R. G. T lymphocytes compartmentalized on the epithelial surface of the lower respiratory tract express the very late activation antigen complex VLA-1. Clin Immunol Immunopathol. 1988 Feb;46(2):221–233. doi: 10.1016/0090-1229(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Shen J. Y., Hofman F. M., Gunter J. R., Modlin R. L., Rea T. H. In situ identification of activated Ta1+ T lymphocytes in human leprosy skin lesions. Int J Lepr Other Mycobact Dis. 1987 Sep;55(3):494–498. [PubMed] [Google Scholar]
- Tabata H., Hara M., Kitani A., Hirose T., Norioka K., Harigai M., Suzuki K., Kawakami M., Kawagoe M., Nakamura H. Expression of TLiSA1 on T cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Immunol Immunopathol. 1989 Sep;52(3):366–375. doi: 10.1016/0090-1229(89)90151-7. [DOI] [PubMed] [Google Scholar]
- Whitaker J. N., Engel W. K. Vascular deposits of immunoglobulin and complement in idiopathic inflammatory myopathy. N Engl J Med. 1972 Feb 17;286(7):333–338. doi: 10.1056/NEJM197202172860701. [DOI] [PubMed] [Google Scholar]
- Williams L. L., Shannon B. T., O'Dougherty M., Wright F. S. Activated T cells in type I Charcot-Marie-Tooth disease: evidence for immunologic heterogeneity. J Neuroimmunol. 1987 Nov;16(3):317–330. doi: 10.1016/0165-5728(87)90108-1. [DOI] [PubMed] [Google Scholar]


