Abstract
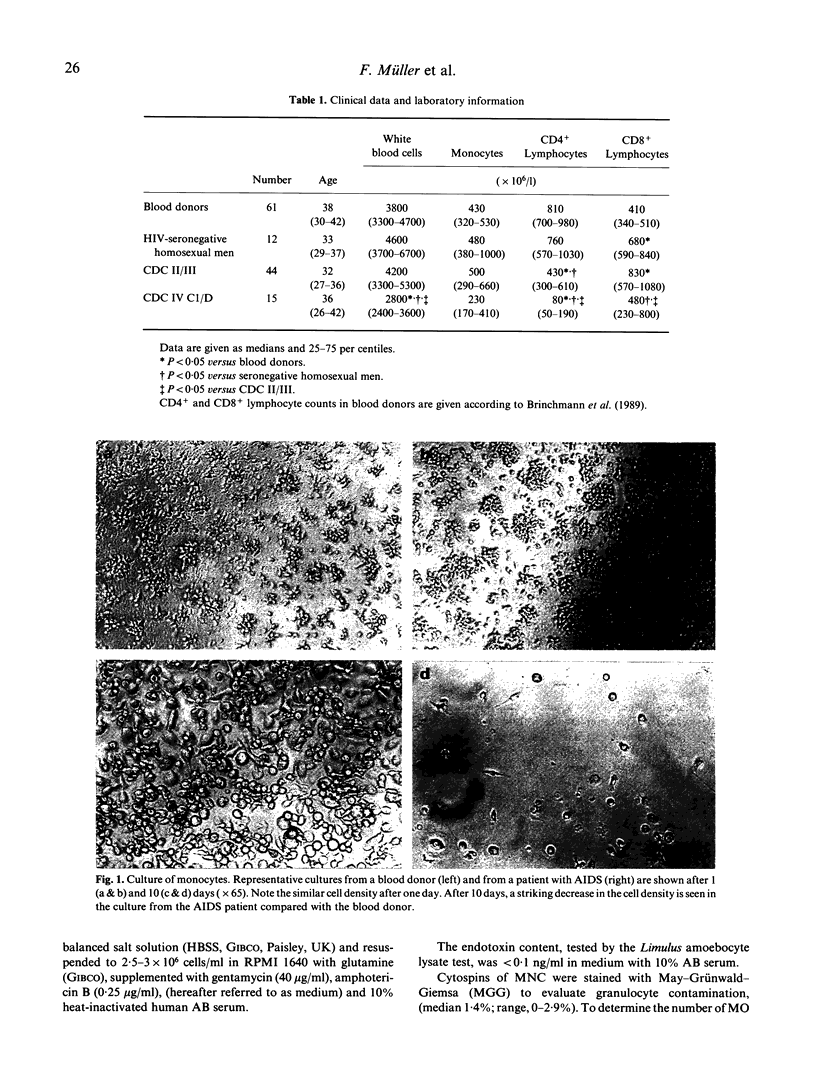
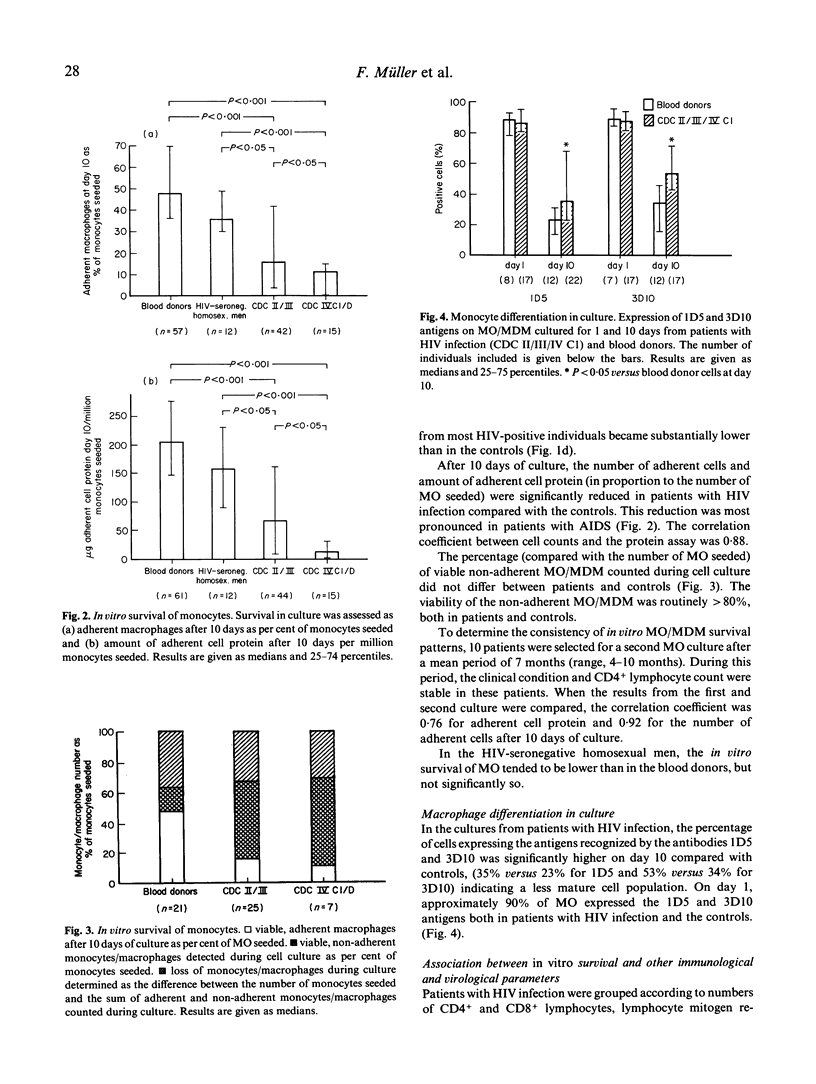
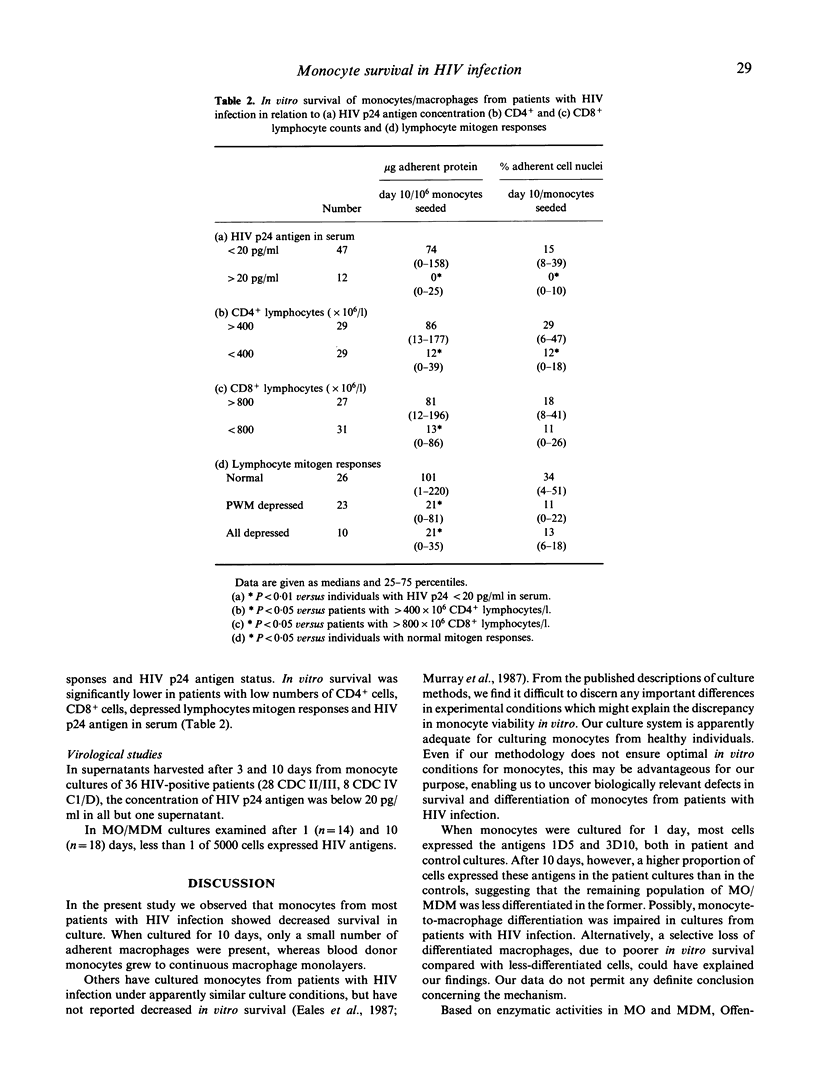
In vitro survival of monocytes (MO) was studied in 59 patients with HIV infection of different clinical stages. MO from 61 donors and 12 healthy seronegative homosexual men were also examined. Compared with the number of MO seeded, the percentage of adherent monocyte-derived macrophages (MDM) present after 10 days was significantly lower in patients with HIV infection than in the controls. However, the number of viable, non-adherent MO/MDM was similar in patients and controls. Our data indicate markedly decreased in vitro survival of MO from patients with HIV infection. After 10 days, the MDM population in the patient cultures was significantly less differentiated than the control cells, assessed by immunocytochemical staining with monoclonal antibodies against differentiation antigens. Reduced in vitro survival of MO/MDM was associated with low numbers of CD4+ and CD8+ lymphocytes in blood, reduced lymphocyte mitogen responses, presence of HIV p24 antigen in serum and advanced clinical stage. Decreased in vitro survival of MO/MDM may be associated with HIV replication in the cells. Although the level of HIV replication in the cultures was low as assessed by measurement of HIV p24 antigen in culture supernatants and staining of MO/MDM for HIV antigens, cytopathogenic effects of HIV or HIV products cannot be ruled out.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentdal O. H., Frøland S. S., Larsen S. Cell-mediated immunity in anorexia nervosa augmented lymphocyte transformation response to concanavalin A and lack of increased risk of infection. Clin Nutr. 1989 Oct;8(5):253–258. doi: 10.1016/0261-5614(89)90035-6. [DOI] [PubMed] [Google Scholar]
- Brinchmann J. E., Vartdal F., Gaudernack G., Markussen G., Funderud S., Ugelstad J., Thorsby E. Direct immunomagnetic quantification of lymphocyte subsets in blood. Clin Exp Immunol. 1988 Jan;71(1):182–186. [PMC free article] [PubMed] [Google Scholar]
- Brinchmann J. E., Vartdal F., Thorsby E. T lymphocyte subset changes in human immunodeficiency virus infection. J Acquir Immune Defic Syndr. 1989;2(4):398–403. [PubMed] [Google Scholar]
- Eales L. J., Moshtael O., Pinching A. J. Microbicidal activity of monocyte derived macrophages in AIDS and related disorders. Clin Exp Immunol. 1987 Feb;67(2):227–235. [PMC free article] [PubMed] [Google Scholar]
- Estevez M. E., Ballart I. J., Diez R. A., Planes N., Scaglione C., Sen L. Early defect of phagocytic cell function in subjects at risk for acquired immunodeficiency syndrome. Scand J Immunol. 1986 Aug;24(2):215–221. doi: 10.1111/j.1365-3083.1986.tb02088.x. [DOI] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Gaudernack G. In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med. 1982 Oct 1;156(4):1101–1114. doi: 10.1084/jem.156.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy J. A., Shimabukuro J., McHugh T., Casavant C., Stites D., Oshiro L. AIDS-associated retroviruses (ARV) can productively infect other cells besides human T helper cells. Virology. 1985 Dec;147(2):441–448. doi: 10.1016/0042-6822(85)90146-1. [DOI] [PubMed] [Google Scholar]
- Maehlen T., Degré M. Lack of activity of fusidic acid against human immunodeficiency virus in monocytes. Antimicrob Agents Chemother. 1989 May;33(5):680–683. doi: 10.1128/aac.33.5.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Scavuzzo D., Jacobs J. L., Kaplan M. H., Libby D. M., Schindler J., Roberts R. B. In vitro and in vivo activation of human mononuclear phagocytes by interferon-gamma. Studies with normal and AIDS monocytes. J Immunol. 1987 Apr 15;138(8):2457–2462. [PubMed] [Google Scholar]
- Müller F., Frøland S. S., Brandtzaeg P. Altered IgG-subclass distribution in lymph node cells and serum of adults infected with human immunodeficiency virus (HIV). Clin Exp Immunol. 1989 Nov;78(2):153–158. [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., Cross G. D., Callaway C. S., McDougal J. S. In vitro infection of human monocytes with human T lymphotropic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV). J Immunol. 1986 Jul 1;137(1):323–329. [PubMed] [Google Scholar]
- Offenberger J., Lieu T., Frick O., Ammann A. J. Impaired monocyte-to-macrophage maturation in patients with lymphadenopathy syndrome. J Clin Immunol. 1986 Nov;6(6):467–471. doi: 10.1007/BF00915252. [DOI] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Bottazzi B., Acero R., Bersani L., Rossi V., Introna M., Lazzarin A., Galli M., Mantovani A. Monocyte function in intravenous drug abusers with lymphadenopathy syndrome and in patients with acquired immunodeficiency syndrome: selective impairment of chemotaxis. Clin Exp Immunol. 1985 Oct;62(1):136–142. [PMC free article] [PubMed] [Google Scholar]
- Roy S., Wainberg M. A. Role of the mononuclear phagocyte system in the development of acquired immunodeficiency syndrome (AIDS). J Leukoc Biol. 1988 Jan;43(1):91–97. doi: 10.1002/jlb.43.1.91. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Smith P. D., Ohura K., Masur H., Lane H. C., Fauci A. S., Wahl S. M. Monocyte function in the acquired immune deficiency syndrome. Defective chemotaxis. J Clin Invest. 1984 Dec;74(6):2121–2128. doi: 10.1172/JCI111637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkeless J. C., Eisen H. N. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J Exp Med. 1975 Dec 1;142(6):1520–1533. doi: 10.1084/jem.142.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. C., Jewett A., Mitsuyasu R., Bonavida B. Spontaneous cytotoxicity and tumor necrosis factor production by peripheral blood monocytes from AIDS patients. J Immunol. 1988 Jul 1;141(1):99–104. [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]



