Abstract
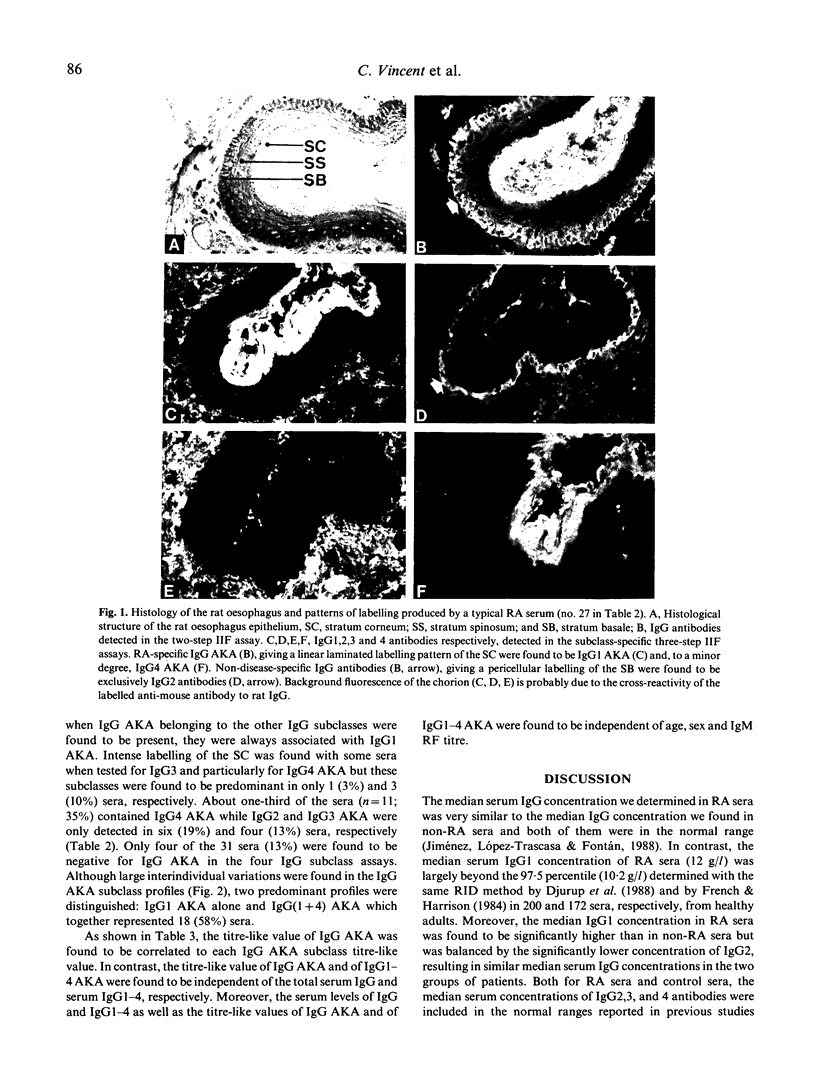
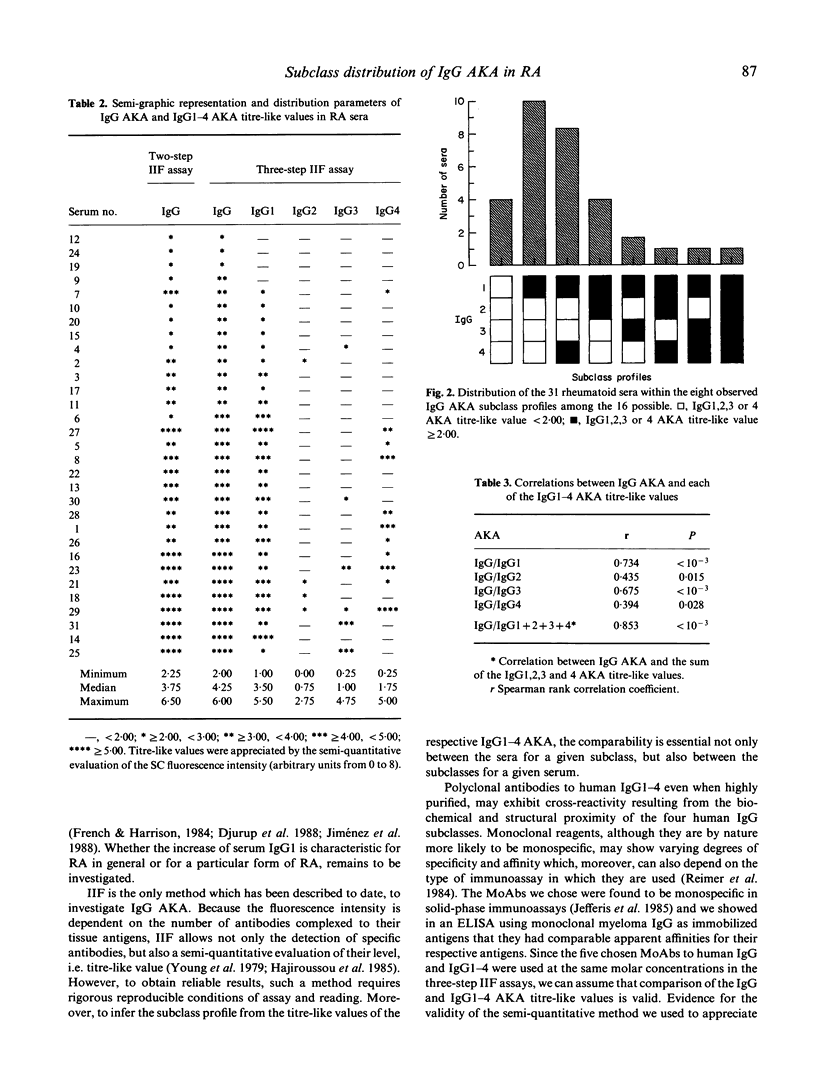
Serum IgG, labelling the stratum corneum of the rat oesophagus epithelium, so-called anti-keratin antibodies (AKA) constitute the most specific marker for the diagnosis of rheumatoid arthritis. In this study, we investigated 31 IgG AKA-positive rheumatoid sera and 21 control sera from patients with non-rheumatoid inflammatory rheumatic diseases. The serum level of IgG1,2,3 and 4 was determined by radial immunodiffusion and the subclass distribution of IgG AKA by a three-step semi-quantitative immunofluorescence assay using standard monoclonal antibodies specific for each of the four human IgG subclasses. In the rheumatoid sera, the serum level of IgG1 was found to be significantly increased and the level of IgG2 significantly decreased with regard to the control sera, while the levels of IgG3 and 4 as well as total IgG were in the normal range. IgG1,2,3, and 4 AKA were detected in 27 (87%), 6 (19%), 4 (13%) and 11 (35%) of the 31 rheumatoid sera, respectively, and were found to be independent of the clinical and biological indices of the disease. In spite of inter-individual heterogeneity, two predominant profiles were distinguished: IgG1 (alone) and IgG(1 + 4), which together represented 18 sera (58%). The large predominance of IgG1 AKA and the quasi-absence of IgG2 AKA suggest that the recognized antigen may be partly comprised of protein. Moreover, the high frequency of occurrence of IgG4 AKA might result from chronic exposure to the eliciting antigen, which could be a genuine autoantigen since we demonstrated that it is also present in the stratum corneum of human epidermis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalberse R. C., van der Gaag R., van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983 Feb;130(2):722–726. [PubMed] [Google Scholar]
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Barrett D. J., Ayoub E. M. IgG2 subclass restriction of antibody to pneumococcal polysaccharides. Clin Exp Immunol. 1986 Jan;63(1):127–134. [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M. Distribution and association of heavy and light chain variable region subgroups among human IgA immunoglobulins. J Immunol. 1975 Feb;114(2 Pt 1):678–681. [PubMed] [Google Scholar]
- Djurup R., Mansa B., Søndergaard I., Weeke B. IgG subclass concentrations in sera from 200 normal adults and IgG subclass determination of 106 myeloma proteins: an interlaboratory study. Scand J Clin Lab Invest. 1988 Feb;48(1):77–83. doi: 10.3109/00365518809085397. [DOI] [PubMed] [Google Scholar]
- French M. A., Harrison G. Serum IgG subclass concentrations in healthy adults: a study using monoclonal antisera. Clin Exp Immunol. 1984 May;56(2):473–475. [PMC free article] [PubMed] [Google Scholar]
- Fukuma N., McLachlan S. M., Petersen V. B., Kau P., Bradbury J., Devey M., Bleasdale K., Grabowski P., Smith B. R. Human thyroglobulin autoantibodies of subclasses IgG2 and IgG4 bind to different epitopes on thyroglobulin. Immunology. 1989 May;67(1):129–131. [PMC free article] [PubMed] [Google Scholar]
- Hajiroussou V. J., Skingle J., Gillett A. P., Webley M. Significance of antikeratin antibodies in rheumatoid arthritis. J Rheumatol. 1985 Feb;12(1):57–59. [PubMed] [Google Scholar]
- Jefferis R., Reimer C. B., Skvaril F., de Lange G., Ling N. R., Lowe J., Walker M. R., Phillips D. J., Aloisio C. H., Wells T. W. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10(3-4):223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Carvalho A., Holborow E. J., Goddard D. H., Russell G. Antiperinuclear factor and keratin antibodies in rheumatoid arthritis. Ann Rheum Dis. 1981 Jun;40(3):263–266. doi: 10.1136/ard.40.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. C., Hamilton R. G., Jordon R. E. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988 Jan;8(1):43–49. doi: 10.1007/BF00915155. [DOI] [PubMed] [Google Scholar]
- Kataaha P. K., Mortazavi-Milani S. M., Russell G., Holborow E. J. Anti-intermediate filament antibodies, antikeratin antibody, and antiperinuclear factor in rheumatoid arthritis and infectious mononucleosis. Ann Rheum Dis. 1985 Jul;44(7):446–449. doi: 10.1136/ard.44.7.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirstein H., Mathiesen F. K. Antikeratin antibodies in rheumatoid arthritis. Methods and clinical significance. Scand J Rheumatol. 1987;16(5):331–338. doi: 10.3109/03009748709102504. [DOI] [PubMed] [Google Scholar]
- Layton G. T., Stanworth D. R. The quantitation of IgG4 antibodies to three common food allergens by ELISA with monoclonal anti-IgG4. J Immunol Methods. 1984 Oct 26;73(2):347–356. doi: 10.1016/0022-1759(84)90410-1. [DOI] [PubMed] [Google Scholar]
- Mergener K., Enzensberger W., Rübsamen-Waigmann H., von Briesen H., Doerr H. W. Immunoglobulin class- and subclass-specific HIV antibody detection in serum and CSF specimens by ELISA and Western blot. Infection. 1987;15(5):317–322. doi: 10.1007/BF01647729. [DOI] [PubMed] [Google Scholar]
- Miossec P., Youinou P., Le Goff P., Moineau M. P. Clinical relevance of antikeratin antibodies in rheumatoid arthritis. Clin Rheumatol. 1982 Sep;1(3):185–189. doi: 10.1007/BF02042772. [DOI] [PubMed] [Google Scholar]
- Morahan G., Berek C., Miller J. F. An idiotypic determinant formed by both immunoglobulin constant and variable regions. Nature. 1983 Feb 24;301(5902):720–722. doi: 10.1038/301720a0. [DOI] [PubMed] [Google Scholar]
- Nys M., Joassin L., Somzee A., Demonty J. Enzyme-linked immunosorbent assay for immunoglobulin G subclass antibodies specific for enterobacterial Re core glycolipid in healthy individuals and in patients infected by gram-negative bacteria. J Clin Microbiol. 1988 May;26(5):857–862. doi: 10.1128/jcm.26.5.857-862.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordeig J., Guardia J. Diagnostic value of antikeratin antibodies in rheumatoid arthritis. J Rheumatol. 1984 Oct;11(5):602–604. [PubMed] [Google Scholar]
- PODLIACHOUK L., EYQUEM A., JACQUELINE F. Le diagnostic de la polyarthrite chronique évolutive par agglutination des globules rouges humains sensibilisés. Ann Inst Pasteur (Paris) 1958 May;94(5):659–662. [PubMed] [Google Scholar]
- Quismorio F. P., Jr, Kaufman R. L., Beardmore T., Mongan E. S. Reactivity of serum antibodies to the keratin layer of rat esophagus in patients with rheumatoid arthritis. Arthritis Rheum. 1983 Apr;26(4):494–499. doi: 10.1002/art.1780260407. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Reimer C. B., Phillips D. J., Aloisio C. H., Moore D. D., Galland G. G., Wells T. W., Black C. M., McDougal J. S. Evaluation of thirty-one mouse monoclonal antibodies to human IgG epitopes. Hybridoma. 1984 Fall;3(3):263–275. doi: 10.1089/hyb.1984.3.263. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Tang F. L., Chan E. K., Pollard K. M., Tsay G., Tan E. M. IgG subclasses of autoantibodies in systemic lupus erythematosus, Sjogren's syndrome, and drug-induced autoimmunity. J Immunol. 1986 Oct 15;137(8):2528–2534. [PubMed] [Google Scholar]
- Schatz D. A., Barrett D. J., Maclaren N. K., Riley W. J. Polyclonal nature of islet cell antibodies in insulin-dependent diabetes. Autoimmunity. 1988;1(1):45–50. doi: 10.3109/08916938808997175. [DOI] [PubMed] [Google Scholar]
- Scott D. L., Delamere J. P., Jones L. J., Walton K. W. Significance of laminar antikeratin antibodies to rat oesophagus in rheumatoid arthritis. Ann Rheum Dis. 1981 Jun;40(3):267–271. doi: 10.1136/ard.40.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre G., Vincent C., Fournié B., Lapeyre F., Soleilhavoup J. P., Fournié A. Anticorps anti-stratum corneum d'oesophage de rat, auto-anticorps anti-kératines épidermiques et anti-épiderme dans la polyarthrite rhumatoïde et différentes affections rhumatologiques. Intérêt diagnostique, aspects fondamentaux. Rev Rhum Mal Osteoartic. 1986 Nov;53(11):607–614. [PubMed] [Google Scholar]
- Serre G., Vincent C., Viraben R., Soleilhavoup J. P. Natural IgM and IgG autoantibodies to epidermal keratins in normal human sera. I: ELISA-titration, immunofluorescence study. J Invest Dermatol. 1987 Jan;88(1):21–27. doi: 10.1111/1523-1747.ep12464810. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Stanescu R., Leibovich S. J. The negative charge of articular cartilage surfaces. Arthritis Rheum. 1986 Apr;29(4):573–573. doi: 10.1002/art.1780290419. [DOI] [PubMed] [Google Scholar]
- Stevens R., Dichek D., Keld B., Heiner D. IgG1 is the predominant subclass of in vivo- and in vitro- produced anti-tetanus toxoid antibodies and also serves as the membrane IgG molecule for delivering inhibitory signals to anti-tetanus toxoid antibody-producing B cells. J Clin Immunol. 1983 Jan;3(1):65–69. doi: 10.1007/BF00919140. [DOI] [PubMed] [Google Scholar]
- Vincent C., Serre G., Lapeyre F., Fournié B., Ayrolles C., Fournié A., Soleilhavoup J. P. High diagnostic value in rheumatoid arthritis of antibodies to the stratum corneum of rat oesophagus epithelium, so-called 'antikeratin antibodies'. Ann Rheum Dis. 1989 Sep;48(9):712–722. doi: 10.1136/ard.48.9.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. J., Mallya R. K., Leslie R. D., Clark C. J., Hamblin T. J. Anti-keratin antibodies in rheumatoid arthritis. Br Med J. 1979 Jul 14;2(6182):97–99. doi: 10.1136/bmj.2.6182.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount W. J., Cohen P., Eisenberg R. A. Distribution of IgG subclasses among human autoantibodies to Sm, RNP, dsDNA, SS-B and IgG rheumatoid factor. Monogr Allergy. 1988;23:41–56. [PubMed] [Google Scholar]