Abstract
Antibody-dependent cell-mediated cytotoxicity (ADCC) has been described in a number of virus infections and occurs in HIV infection. In spite of numerous studies on the ability of HIV-positive sera to mediate ADCC, it remains unclear whether ADCC has any functional significance. Here we examine serial serum samples from a cohort of haemophilia patients who became infected from a common source in 1984, and show that there is no significant difference in ADCC values between those who remained asymptomatic and those who progressed to disease. This study does not compare effector cell function and clinical status and does not exclude activity against different target cell lineages in vivo.
Full text
PDF
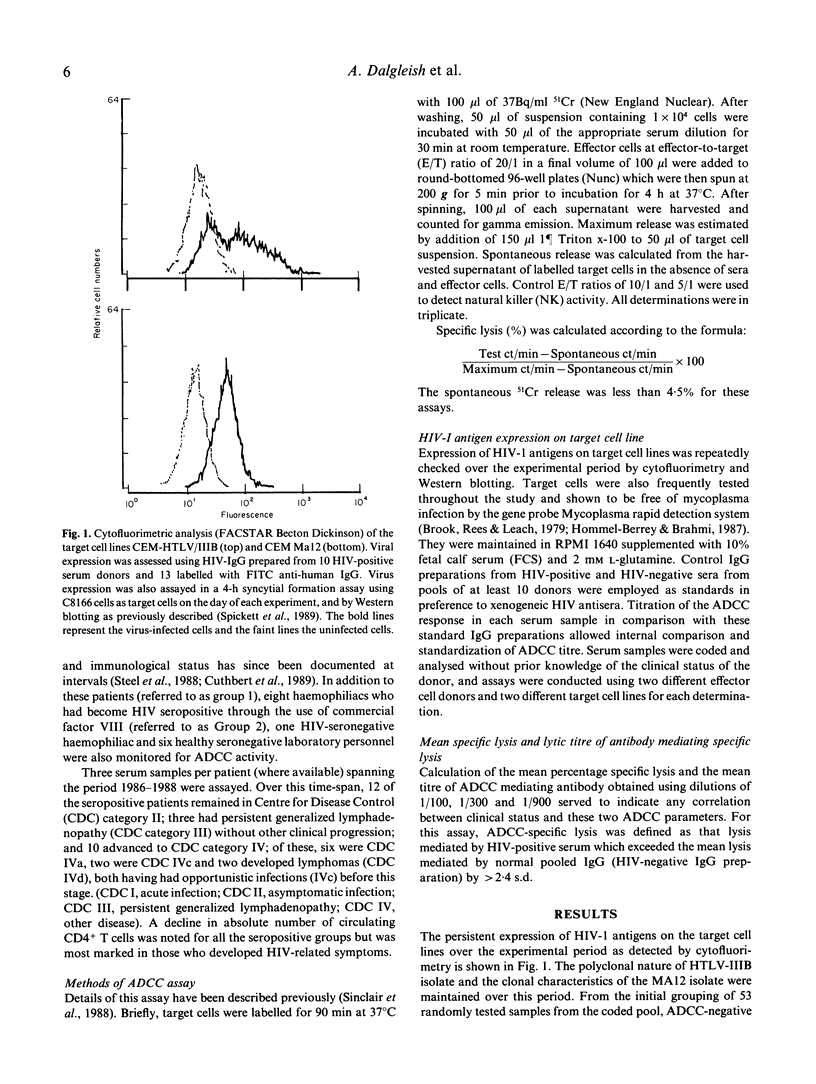
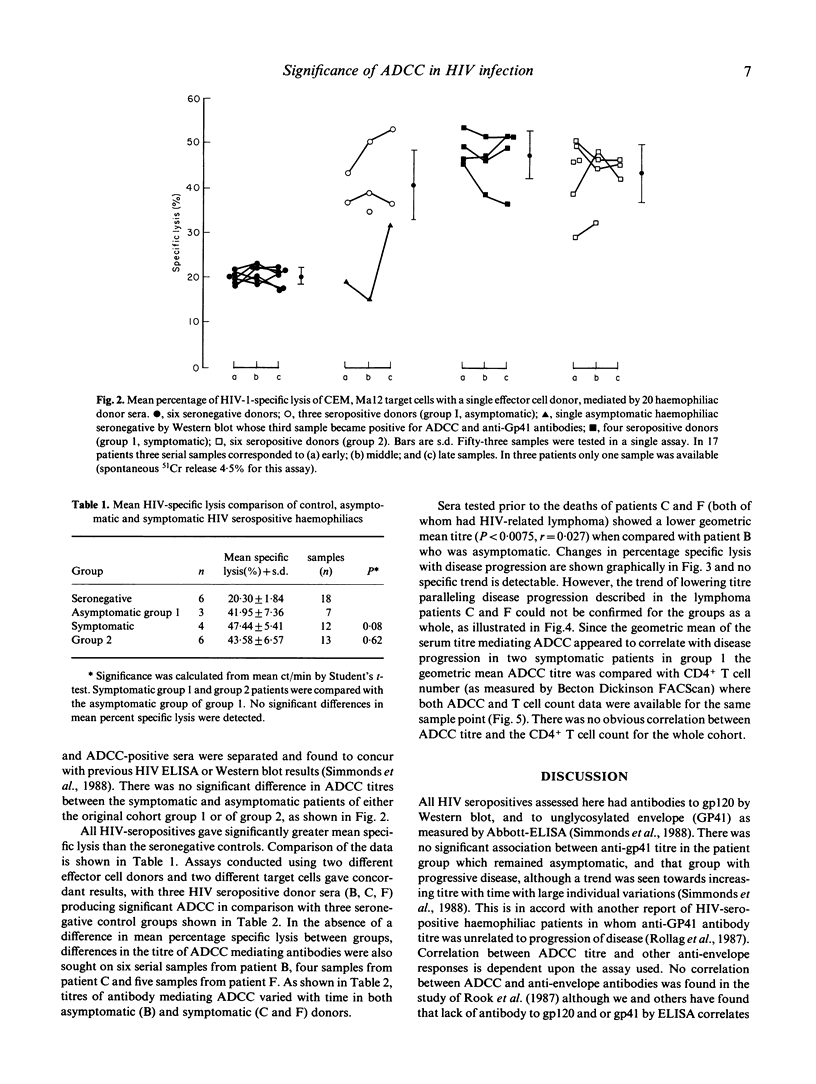
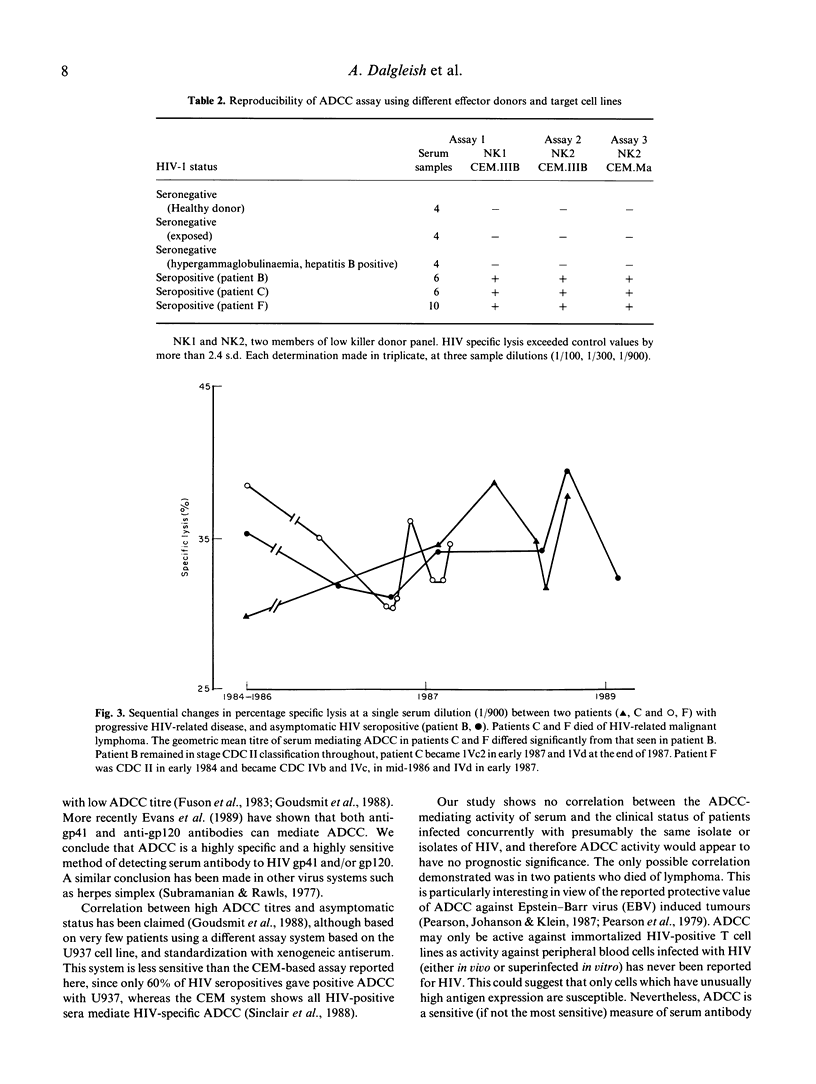
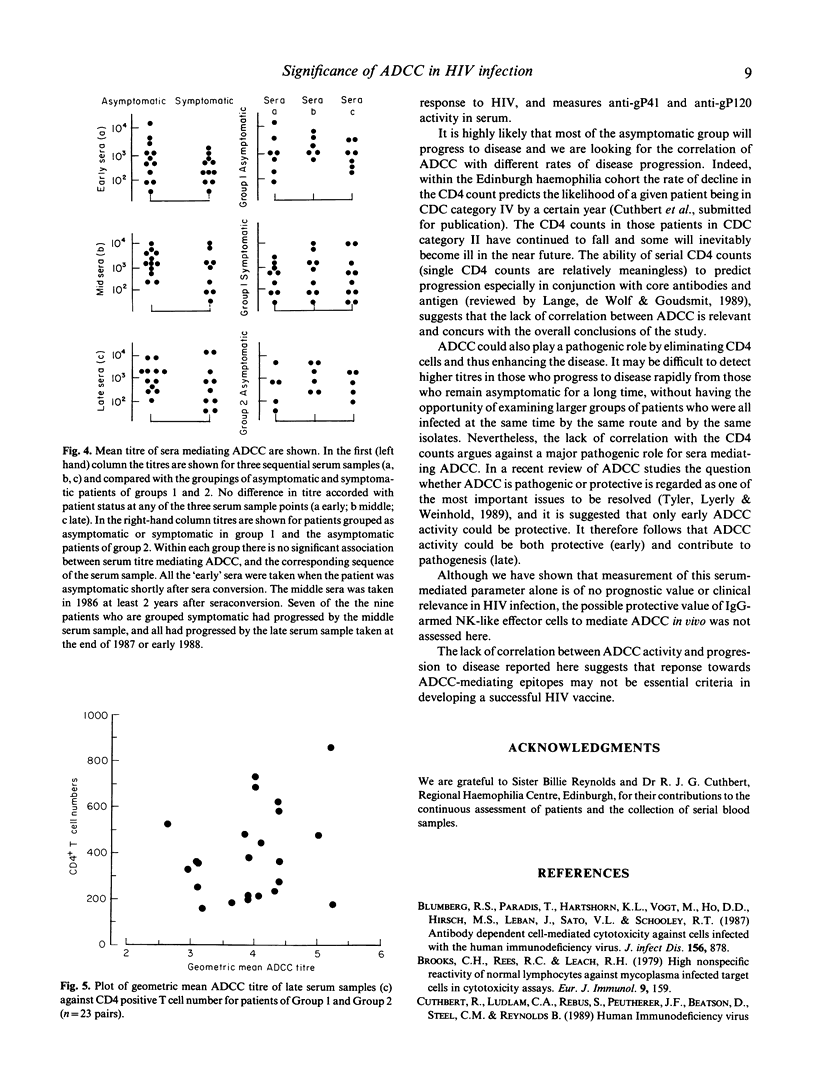

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg R. S., Paradis T., Hartshorn K. L., Vogt M., Ho D. D., Hirsch M. S., Leban J., Sato V. L., Schooley R. T. Antibody-dependent cell-mediated cytotoxicity against cells infected with the human immunodeficiency virus. J Infect Dis. 1987 Dec;156(6):878–884. doi: 10.1093/infdis/156.6.878. [DOI] [PubMed] [Google Scholar]
- Brooks C. G., Rees R. C., Leach R. H. High nonspecific reactivity of normal lymphocytes against mycoplasma-infected target cells in cytotoxicity assays. Eur J Immunol. 1979 Feb;9(2):159–165. doi: 10.1002/eji.1830090213. [DOI] [PubMed] [Google Scholar]
- Evans L. A., Thomson-Honnebier G., Steimer K., Paoletti E., Perkus M. E., Hollander H., Levy J. A. Antibody-dependent cellular cytotoxicity is directed against both the gp120 and gp41 envelope proteins of HIV. AIDS. 1989 May;3(5):273–276. doi: 10.1097/00002030-198905000-00004. [DOI] [PubMed] [Google Scholar]
- Fanger M. W., Shen L., Graziano R. F., Guyre P. M. Cytotoxicity mediated by human Fc receptors for IgG. Immunol Today. 1989 Mar;10(3):92–99. doi: 10.1016/0167-5699(89)90234-X. [DOI] [PubMed] [Google Scholar]
- Fuson E. W., Hubbard R. A., Sugantharaj D. G., Andrews R. B., Beard M. R., Whittaker R. L. Antibody-dependent cell-mediated cytotoxicity. Effectors, signals, and mechanisms. Surv Immunol Res. 1983;2(4):327–340. doi: 10.1007/BF02918449. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Ljunggren K., Smit L., Jondal M., Fenyö E. M., Jonda M. Biological significance of the antibody response to HIV antigens expressed on the cell surface. Arch Virol. 1988;103(3-4):189–206. doi: 10.1007/BF01311092. [DOI] [PubMed] [Google Scholar]
- Hommel-Berrey G. A., Brahmi Z. Relevance of soluble cytotoxic factors generated by mycoplasma-contaminated targets to natural killer cell-mediated killing. Hum Immunol. 1987 Sep;20(1):33–46. doi: 10.1016/0198-8859(87)90004-8. [DOI] [PubMed] [Google Scholar]
- Howell D. N., Andreotti P. E., Dawson J. R., Cresswell P. Natural killing target antigens as inducers of interferon: studies with an immunoselected, natural killing-resistant human T lymphoblastoid cell line. J Immunol. 1985 Feb;134(2):971–976. [PubMed] [Google Scholar]
- Koup R. A., Sullivan J. L., Levine P. H., Brewster F., Mahr A., Mazzara G., McKenzie S., Panicali D. Antigenic specificity of antibody-dependent cell-mediated cytotoxicity directed against human immunodeficiency virus in antibody-positive sera. J Virol. 1989 Feb;63(2):584–590. doi: 10.1128/jvi.63.2.584-590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange J. M., de Wolf F., Goudsmit J. Markers for progression in HIV infection. AIDS. 1989;3 (Suppl 1):S153–S160. doi: 10.1097/00002030-198901001-00023. [DOI] [PubMed] [Google Scholar]
- Ljunggren K., Böttiger B., Biberfeld G., Karlson A., Fenyö E. M., Jondal M. Antibody-dependent cellular cytotoxicity-inducing antibodies against human immunodeficiency virus. Presence at different clinical stages. J Immunol. 1987 Oct 1;139(7):2263–2267. [PubMed] [Google Scholar]
- Ljunggren K., Fenyö E. M., Biberfeld G., Jondal M. Detection of antibodies which mediate human immunodeficiency virus-specific cellular cytotoxicity (ADCC) in vitro. J Immunol Methods. 1987 Nov 23;104(1-2):7–14. doi: 10.1016/0022-1759(87)90481-9. [DOI] [PubMed] [Google Scholar]
- Ludlam C. A., Tucker J., Steel C. M., Tedder R. S., Cheingsong-Popov R., Weiss R. A., McClelland D. B., Philp I., Prescott R. J. Human T-lymphotropic virus type III (HTLV-III) infection in seronegative haemophiliacs after transfusion of factor VIII. Lancet. 1985 Aug 3;2(8449):233–236. doi: 10.1016/s0140-6736(85)90288-0. [DOI] [PubMed] [Google Scholar]
- Lyerly H. K., Matthews T. J., Langlois A. J., Bolognesi D. P., Weinhold K. J. Human T-cell lymphotropic virus IIIB glycoprotein (gp120) bound to CD4 determinants on normal lymphocytes and expressed by infected cells serves as target for immune attack. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4601–4605. doi: 10.1073/pnas.84.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Nishanian P., Keith D. E., Jr, Houghton R. L., Heitjan D. F., Fahey J. L., Giorgi J. V. Antibodies to human immunodeficiency virus in human sera induce cell-mediated lysis of human immunodeficiency virus-infected cells. J Immunol. 1987 Oct 1;139(7):2458–2463. [PubMed] [Google Scholar]
- Pearson G. R., Johansson B., Klein G. Antibody-dependent cellular cytotoxicity against Epstein-Barr virus-associated antigens in African patients with nasopharyngeal carcinoma. Int J Cancer. 1978 Aug 15;22(2):120–125. doi: 10.1002/ijc.2910220203. [DOI] [PubMed] [Google Scholar]
- Pearson G. R., Qualtiere L. F., Klein G., Norin T., Bal I. S. Epstein-Barr virus-specific antibody-dependent cellular cytotoxicity in patients with Burkitt's lymphoma. Int J Cancer. 1979 Oct 15;24(4):402–406. doi: 10.1002/ijc.2910240405. [DOI] [PubMed] [Google Scholar]
- Robert-Guroff M., Brown M., Gallo R. C. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature. 1985 Jul 4;316(6023):72–74. doi: 10.1038/316072a0. [DOI] [PubMed] [Google Scholar]
- Rollag H., Evensen S. A., Frøland S. S., Glomstein A. The significance of HIV antigen and HIV antibodies specific against envelope and core proteins in serum of HIV-infected hemophiliacs. Eur J Haematol. 1987 Oct;39(4):353–355. doi: 10.1111/j.1600-0609.1987.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Rook A. H., Lane H. C., Folks T., McCoy S., Alter H., Fauci A. S. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol. 1987 Feb 15;138(4):1064–1067. [PubMed] [Google Scholar]
- Simmonds P., Lainson F. A., Cuthbert R., Steel C. M., Peutherer J. F., Ludlam C. A. HIV antigen and antibody detection: variable responses to infection in the Edinburgh haemophiliac cohort. Br Med J (Clin Res Ed) 1988 Feb 27;296(6622):593–598. doi: 10.1136/bmj.296.6622.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A. L., Habeshaw J. A., Muir L., Chandler P., Forster S., Cruickshank K., Dalgleish A. G. Antibody-dependent cell-mediated cytotoxicity: comparison between HTLV-I and HIV-1 assays. AIDS. 1988 Dec;2(6):465–472. [PubMed] [Google Scholar]
- Spickett G., Beattie R. E., Bountiff L., Dalgleish A. G., Webster A. D. Quantitation of HIV-1 activity in tissue culture supernatants: effects of culture condition on syncytial assays and virus production. J Virol Methods. 1989 Apr-May;24(1-2):67–76. doi: 10.1016/0166-0934(89)90008-6. [DOI] [PubMed] [Google Scholar]
- Steel C. M., Ludlam C. A., Beatson D., Peutherer J. F., Cuthbert R. J., Simmonds P., Morrison H., Jones M. HLA haplotype A1 B8 DR3 as a risk factor for HIV-related disease. Lancet. 1988 May 28;1(8596):1185–1188. doi: 10.1016/s0140-6736(88)92009-0. [DOI] [PubMed] [Google Scholar]
- Subramanian T., Rawls W. E. Comparison of antibody-dependent cellular cytotoxicity and complement-dependent antibody lysis of herpes simplex virus-infected cells as methods of detecting antiviral antibodies in human sera. J Clin Microbiol. 1977 Jun;5(6):551–558. doi: 10.1128/jcm.5.6.551-558.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. S., Lyerly H. K., Weinhold K. J. Anti-HIV-1 ADCC. AIDS Res Hum Retroviruses. 1989 Dec;5(6):557–563. doi: 10.1089/aid.1989.5.557. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P. R., Cheingsong-Popov R., Dalgleish A. G., Carne C. A., Weller I. V., Tedder R. S. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature. 1985 Jul 4;316(6023):69–72. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P. R., Weber J. N., Dalgleish A. G., Lasky L. A., Berman P. W. Variable and conserved neutralization antigens of human immunodeficiency virus. Nature. 1986 Dec 11;324(6097):572–575. doi: 10.1038/324572a0. [DOI] [PubMed] [Google Scholar]
- de la Barrera S., Fainboim L., Lugo S., Picchio G. R., Muchinik G. R., de Bracco M. M. Anti-class II antibodies in AIDS patients and AIDS-risk groups. Immunology. 1987 Dec;62(4):599–604. [PMC free article] [PubMed] [Google Scholar]


