Abstract
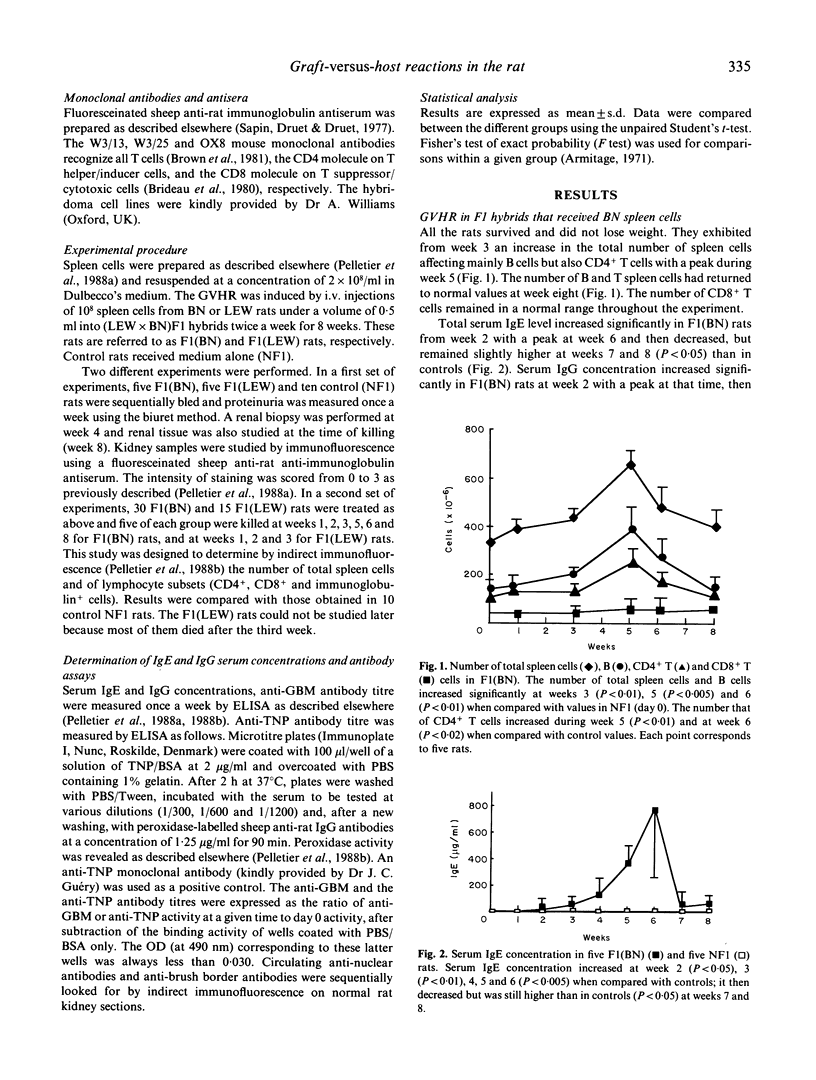
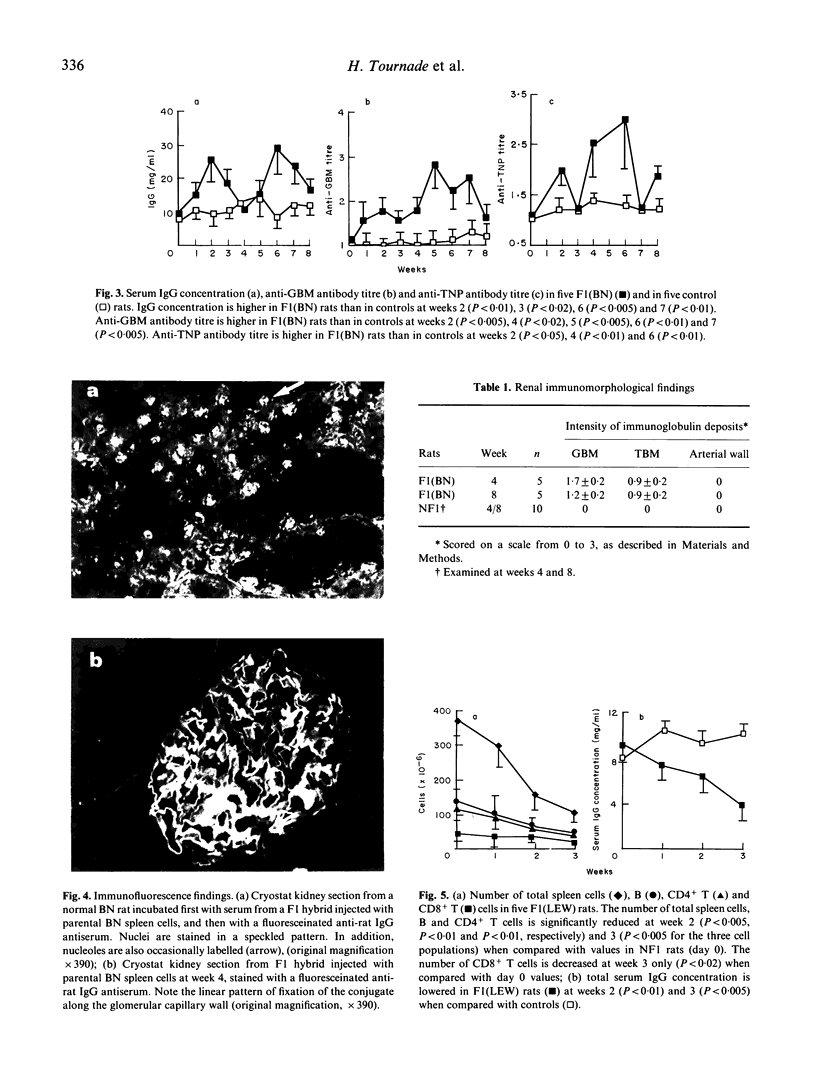
Gold salts, D-penicillamine or mercurials induce autoimmunity in Brown Norway (BN) rats and provoke an immunosuppression in Lewis (LEW) rats. It has been suggested that immunologically mediated manifestations induced by drugs could result from graft-versus-host (GVH) like reactions. We show that BN spleen cells transferred into (LEW x BN)F1 hybrids induce a chronic GVH reaction (GVHR). This reaction led to an autoimmune disease quite similar to that induced by drugs in BN rats. In both situations, a common part of the B cell repertoire is triggered. In contrast, LEW spleen cells transferred into (LEW x BN)F1 hybrids provoke a lethal GVHR. This is to be compared with the CD8-mediated immunosuppression observed in LEW rats injected with HgCl2. These findings are in agreement with the prediction that immune dysregulation induced by drugs leads to GVH-like reactions either stimulatory or suppressive depending upon the strain tested.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellon B., Capron M., Druet E., Verroust P., Vial M. C., Sapin C., Girard J. F., Foidart J. M., Mahieu P., Druet P. Mercuric chloride induced autoimmune disease in Brown-Norway rats: sequential search for anti-basement membrane antibodies and circulating immune complexes. Eur J Clin Invest. 1982 Apr;12(2):127–133. doi: 10.1111/j.1365-2362.1982.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Bowman C., Mason D. W., Pusey C. D., Lockwood C. M. Autoregulation of autoantibody synthesis in mercuric chloride nephritis in the Brown Norway rat. I. A role for T suppressor cells. Eur J Immunol. 1984 May;14(5):464–470. doi: 10.1002/eji.1830140515. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Chalopin J. M., Lockwood C. M. Autoregulation of autoantibody synthesis in mercuric chloride nephritis in the Brown Norway rat. II. Presence of antigen-augmentable plaque-forming cells in the spleen is associated with humoral factors behaving as auto-anti-idiotypic antibodies. Eur J Immunol. 1984 May;14(5):470–475. doi: 10.1002/eji.1830140516. [DOI] [PubMed] [Google Scholar]
- Donker A. J., Venuto R. C., Vladutiu A. O., Brentjens J. R., Andres G. A. Effects of prolonged administration of D-penicillamine or captopril in various strains of rats. Brown Norway rats treated with D-penicillamine develop autoantibodies, circulating immune complexes, and disseminated intravascular coagulation. Clin Immunol Immunopathol. 1984 Jan;30(1):142–155. doi: 10.1016/0090-1229(84)90015-1. [DOI] [PubMed] [Google Scholar]
- Druet P., Baran D., Pelletier L., Hirsch F., Druet E., Sapin C. Drug-induced experimental autoimmune nephritis. Concepts Immunopathol. 1986;3:311–330. [PubMed] [Google Scholar]
- Gleichmann E., Gleichmann H., Wilke W. Autoimmunization and lymphomagenesis in parent to F1 combinations differing at the major histocompatibility complex: model for spontaneous disease caused by altered self-antigens? Transplant Rev. 1976;31:156–224. doi: 10.1111/j.1600-065x.1976.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Gleichmann E., Van Elven E. H., Van der Veen J. P. A systemic lupus erythematosus (SLE)-like disease in mice induced by abnormal T-B cell cooperation. Preferential formation of autoantibodies characteristic of SLE. Eur J Immunol. 1982 Feb;12(2):152–159. doi: 10.1002/eji.1830120210. [DOI] [PubMed] [Google Scholar]
- Guéry J. C., Tournade H., Pelletier L., Druet E., Druet P. Rat anti-glomerular basement membrane antibodies in toxin-induced autoimmunity and in chronic graft-vs.-host reaction share recurrent idiotypes. Eur J Immunol. 1990 Jan;20(1):101–105. doi: 10.1002/eji.1830200115. [DOI] [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Pals S. T., Radaszkiewicz T., Gleichmann E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs-host disease. IV. Activation of donor allosuppressor cells is confined to acute GVHD. J Immunol. 1984 Apr;132(4):1669–1678. [PubMed] [Google Scholar]
- Parkman R. Graft-versus-host disease: an alternative hypothesis. Immunol Today. 1989 Nov;10(11):362–364. doi: 10.1016/0167-5699(89)90267-3. [DOI] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Guettier C., Vial M. C., Mandet C., Nochy D., Bazin H., Druet P. HgC12 induces T and B cells to proliferate and differentiate in BN rats. Clin Exp Immunol. 1988 Feb;71(2):336–342. [PMC free article] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Hirsch F., Sapin C., Druet P. Autoreactive T cells in mercury-induced autoimmune disease: in vitro demonstration. J Immunol. 1986 Oct 15;137(8):2548–2554. [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Rossert J., Druet P. HgCl2 induces nonspecific immunosuppression in Lewis rats. Eur J Immunol. 1987 Jan;17(1):49–54. doi: 10.1002/eji.1830170109. [DOI] [PubMed] [Google Scholar]
- Pelletier L., Pasquier R., Rossert J., Vial M. C., Mandet C., Druet P. Autoreactive T cells in mercury-induced autoimmunity. Ability to induce the autoimmune disease. J Immunol. 1988 Feb 1;140(3):750–754. [PubMed] [Google Scholar]
- Prouvost-Danon A., Abadie A., Sapin C., Bazin H., Druet P. Induction of IgE synthesis and potentiation of anti-ovalbumin IgE antibody response by HgCl2 in the rat. J Immunol. 1981 Feb;126(2):699–792. [PubMed] [Google Scholar]
- Robinson C. J., Balazs T., Egorov I. K. Mercuric chloride-, gold sodium thiomalate-, and D-penicillamine-induced antinuclear antibodies in mice. Toxicol Appl Pharmacol. 1986 Nov;86(2):159–169. doi: 10.1016/0041-008x(86)90046-3. [DOI] [PubMed] [Google Scholar]
- Rolink A. G., Gleichmann E. Allosuppressor- and allohelper-T cells in acute and chronic graft-vs.-host (GVH) disease. III. Different Lyt subsets of donor T cells induce different pathological syndromes. J Exp Med. 1983 Aug 1;158(2):546–558. doi: 10.1084/jem.158.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossert J., Pelletier L., Pasquier R., Druet P. Autoreactive T cells in mercury-induced autoimmunity. Demonstration by limiting dilution analysis. Eur J Immunol. 1988 Nov;18(11):1761–1766. doi: 10.1002/eji.1830181116. [DOI] [PubMed] [Google Scholar]
- Sapin C., Druet E., Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977 Apr;28(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Shiohara T., Narimatsu H., Nagashima M. Induction of cutaneous graft-versus-host disease by allo- or self-Ia-reactive helper T cells in mice. Transplantation. 1987 May;43(5):692–698. doi: 10.1097/00007890-198705000-00018. [DOI] [PubMed] [Google Scholar]
- Tournade H., Pelletier L., Pasquier R., Vial M. C., Mandet C., Druet P. D-penicillamine-induced autoimmunity in Brown-Norway rats. Similarities with HgCl2-induced autoimmunity. J Immunol. 1990 Apr 15;144(8):2985–2991. [PubMed] [Google Scholar]
- Via C. S., Sharrow S. O., Shearer G. M. Role of cytotoxic T lymphocytes in the prevention of lupus-like disease occurring in a murine model of graft-vs-host disease. J Immunol. 1987 Sep 15;139(6):1840–1849. [PubMed] [Google Scholar]
- Via C. S., Shearer G. M. T-cell interactions in autoimmunity: insights from a murine model of graft-versus-host disease. Immunol Today. 1988 Jul-Aug;9(7-8):207–213. doi: 10.1016/0167-5699(88)91215-7. [DOI] [PubMed] [Google Scholar]
- van Rappard-van der Veen F. M., Rolink A. G., Gleichmann E. Diseases caused by reactions of T lymphocytes towards incompatible structures of the major histocompatibility complex. VI. Autoantibodies characteristic of systemic lupus erythematosus induced by abnormal T-B cell cooperation across I-E. J Exp Med. 1982 May 1;155(5):1555–1560. doi: 10.1084/jem.155.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]


