Abstract
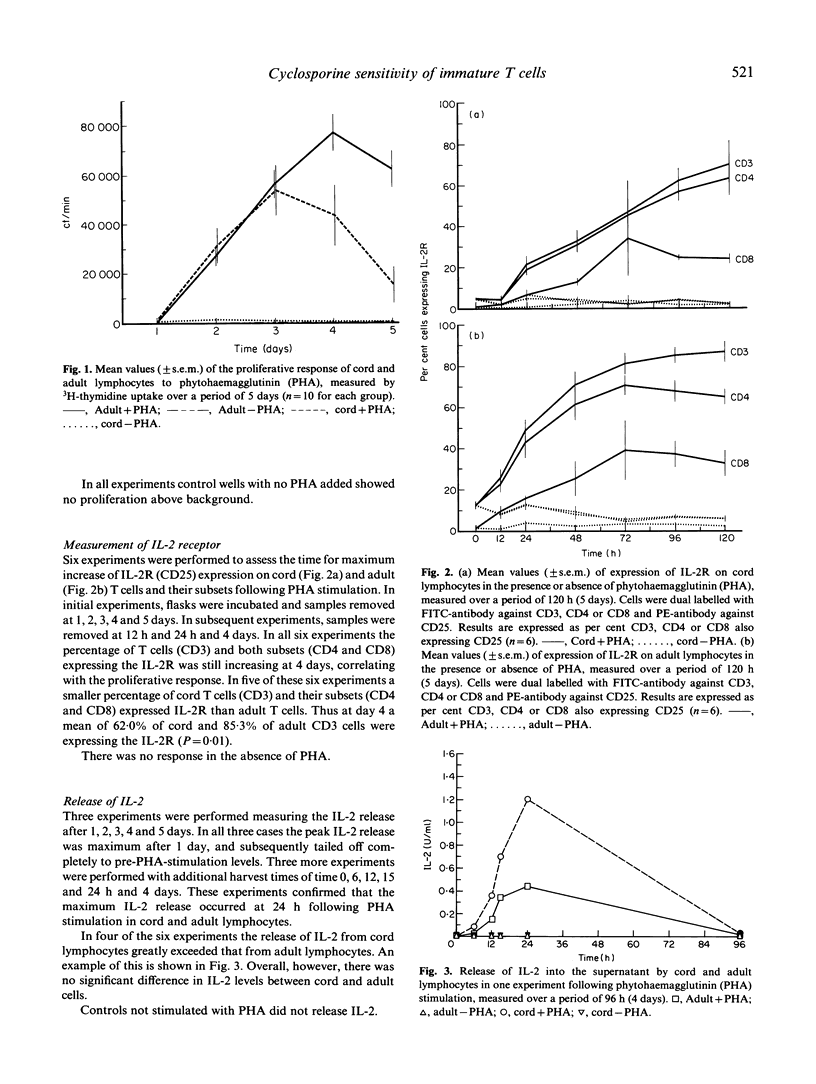
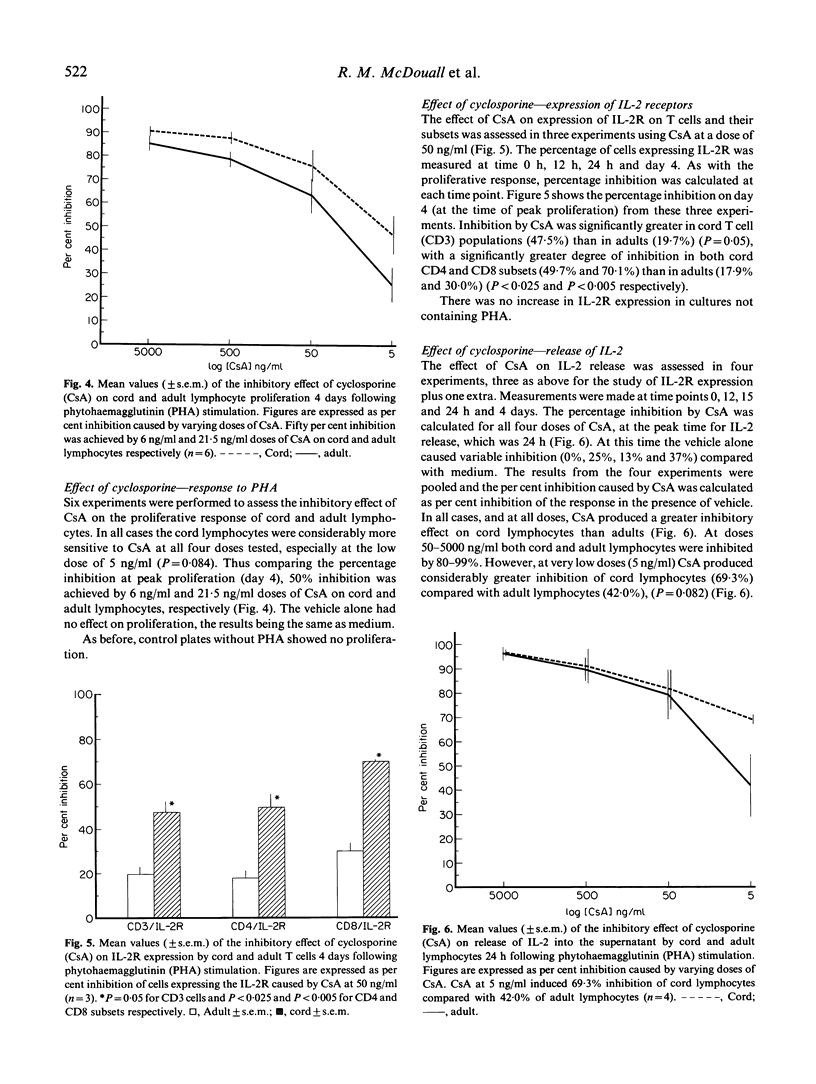
Despite the increasing numbers of paediatric transplants performed, little is known about the immune responses of T lymphocytes in human neonates. Here we have compared the effects of cyclosporine on the phytohaemagglutinin (PHA) response of immature (cord) and mature (adult) lymphocytes using the following parameters of activation: (i) proliferation, measured by 3H-thymidine uptake; (ii) expression of cell surface IL-2 receptor; (iii) release of IL-2 into the supernatant. Cyclosporine was added to cultures of PHA-stimulated lymphocytes at doses ranging from 5 to 5000 ng/ml. The proliferative response of cord lymphocytes was considerably more sensitive to cyclosporine at each dose, so that 50% inhibition was achieved by 6 ng/ml and 21.5 ng/ml doses of cyclosporine on cord and adult lymphocytes, respectively. Expression of the IL-2 receptor by PHA-activated T cells and their subsets was assessed by flow cytometry. Cyclosporine inhibited IL-2 receptor expression to a significantly greater degree in cord CD4 and CD8 cells (49.7% and 70.1%) than in adults (17.9% and 30.0%). Biologically active IL-2 release was measured using the IL-2-dependent cell line CTLL-2. Cyclosporine at doses 50-5000 ng/ml produced 80-99% inhibition of both cord and adult responses. However, at very low doses (5 ng/ml) cyclosporine produced 69.3% inhibition of cord lymphocytes, compared with 42.0% of adult lymphocytes. These results suggest that the T cells of neonates are considerably more sensitive to cyclosporine than are adult T cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson U., Bird A. G., Britton B. S., Palacios R. Humoral and cellular immunity in humans studied at the cell level from birth to two years of age. Immunol Rev. 1981;57:1–38. doi: 10.1111/j.1600-065x.1981.tb00440.x. [DOI] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bettens F., Walker C., Bonnard G. D., de Weck A. L. Effect of cyclosporin A on the early activation of human T helper lymphocytes: inhibition of RNA-synthesis and modification of the expression of activation antigens. Immunobiology. 1985 Dec;170(5):434–447. doi: 10.1016/S0171-2985(85)80067-X. [DOI] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Krönke M., Noguchi P. D., Cunningham R. E., Waldmann T. A., Greene W. C. Regulation of interleukin 2 receptor expression: effects of phorbol diester, phospholipase C, and reexposure to lectin or antigen. J Immunol. 1984 Dec;133(6):3054–3061. [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Fairfax C. A., Borzy M. S. Interleukin 2 production, proliferative response, and receptor expression by cord blood mononuclear cells. J Clin Lab Immunol. 1988 Oct;27(2):63–67. [PubMed] [Google Scholar]
- Foa R., Giubellino M. C., Fierro M. T., Lusso P., Ferrando M. L. Immature T lymphocytes in human cord blood identified by monoclonal antibodies: a model for the study of the differentiation pathway of T cells in humans. Cell Immunol. 1984 Nov;89(1):194–201. doi: 10.1016/0008-8749(84)90209-0. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Kurnick J. Newborn T cell suppression: early appearance, maintenance in culture, and lack of growth factor suppression. J Immunol. 1981 Jan;126(1):50–53. [PubMed] [Google Scholar]
- Hess A. D., Esa A. H., Colombani P. M. Mechanisms of action of cyclosporine: effect on cells of the immune system and on subcellular events in T cell activation. Transplant Proc. 1988 Apr;20(2 Suppl 2):29–40. [PubMed] [Google Scholar]
- Kawano Y., Noma T., Yata J. Analysis of decreased autologous mixed lymphocyte reaction of cord blood lymphocytes: with special reference to production of and response to interleukin-2 (IL-2). Asian Pac J Allergy Immunol. 1984 Jun;2(1):49–55. [PubMed] [Google Scholar]
- Kay H. E., Doe J., Hockley A. Response of human foetal thymocytes to phytohaemagglutinin (PHA). Immunology. 1970 Mar;18(3):393–396. [PMC free article] [PubMed] [Google Scholar]
- Li B., Sehajpal P. K., Subramaniam A., Joseph A., Stenzel K. H., Suthanthiran M. Inhibition of interleukin 2 receptor expression in normal human T cells by cyclosporine. Demonstration at the mRNA, protein, and functional levels. Transplantation. 1992 Jan;53(1):146–151. doi: 10.1097/00007890-199201000-00030. [DOI] [PubMed] [Google Scholar]
- Lin C. S., Boltz R. C., Siekierka J. J., Sigal N. H. FK-506 and cyclosporin A inhibit highly similar signal transduction pathways in human T lymphocytes. Cell Immunol. 1991 Apr 1;133(2):269–284. doi: 10.1016/0008-8749(91)90103-i. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Seki H., Taga K., Sato H., Taniguchi N. Dissociated production of interleukin-2 and immune (gamma) interferon by phytohaemagglutinin stimulated lymphocytes in healthy infants. Clin Exp Immunol. 1985 Feb;59(2):505–511. [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T., Yachie A., Ohzeki S., Nagaoki T., Taniguchi N. Cyclosporin A does not prevent expression of Tac antigen, a probable TCGF receptor molecule, on mitogen-stimulated human T cells. J Immunol. 1983 Jun;130(6):2737–2742. [PubMed] [Google Scholar]
- Nelson D. L., Kurman C. C., Fritz M. E., Boutin B., Rubin L. A. The production of soluble and cellular interleukin-2 receptors by cord blood mononuclear cells following in vitro activation. Pediatr Res. 1986 Feb;20(2):136–139. doi: 10.1203/00006450-198602000-00008. [DOI] [PubMed] [Google Scholar]
- Rayfield L. S., Brent L., Rodeck C. H. Development of cell-mediated lympholysis in human foetal blood lymphocytes. Clin Exp Immunol. 1980 Dec;42(3):561–570. [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- Tsao P. W., Diaz R. J., Radde I. C., Wong P. Y., Martell M. F., Augustine J. M., Wilson G. J., Coles J. G. Age-related differences in the effects of cyclosporine on lymphocyte intracellular free calcium. Transplantation. 1991 Aug;52(2):345–349. doi: 10.1097/00007890-199108000-00031. [DOI] [PubMed] [Google Scholar]
- Weir M. R., Peppler R., Gomolka D., Handwerger B. S. Additive inhibition of afferent and efferent immunological responses of human peripheral blood mononuclear cells by verapamil and cyclosporine. Transplantation. 1991 Apr;51(4):851–857. doi: 10.1097/00007890-199104000-00022. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Westall J., Johnston L., Lewis D. B., Dower S. K., Alpert A. R. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J Clin Invest. 1986 Mar;77(3):860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. Y., Lawton A. R., Cooper M. D. Differentiation capacity of cultured B lymphocytes from immunodeficient patients. J Clin Invest. 1973 Dec;52(12):3180–3189. doi: 10.1172/JCI107518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi T., Miyawaki T., Yachie A., Ohzeki S., Taniguchi N. Discrepancy in expression ability of Tac antigen and Ia determinants defined by monoclonal antibodies on activated or cultured cord blood T lymphocytes. J Immunol. 1982 Oct;129(4):1441–1445. [PubMed] [Google Scholar]


