Abstract
Transforming growth factor beta-1 (TGF-beta) is a multi-potent immunoregulatory peptide that has effects on numerous cell types. Here we report that human TGF-beta inhibits the activation of the macrophage cell line RAW 264.7 for killing of the L1210 tumour cell line. RAW 264.7 cells, like normal macrophages, require sequential interaction with priming and triggering stimuli for full activation of cytolytic activity. TGF-beta inhibits this cytotoxicity in a dose-dependent manner at both the priming and the triggering stage. Addition of as little as 1 ng/ml TGF-beta when added with either the priming signal, recombinant interferon-gamma (IFN-gamma), or the triggering signal, bacterial lipopolysaccharide (LPS), completely abrogated tumouricidal activity. Incubation with TGF-beta also inhibited the morphological changes normally observed in activated RAW 264.7 cells. However, TGF-beta was unable to inhibit the cytotoxic activity of RAW 264.7 cells against the target cell line WEHI 164, which is sensitive to tumour necrosis factor. In contrast to the effects on cytotoxic activity, the cytostatic activity of activated RAW 264.7 cells was not inhibited by TGF-beta at doses of up to 5 ng/ml. In addition, pretreatment of the L1210 target cells with TGF-beta made them refractory to both the cytostatic and cytotoxic effects of RAW 264.7 cells. These data suggest that TGF-beta may be an important mediator in the regulation of macrophage tumouricidal activity.
Full text
PDF

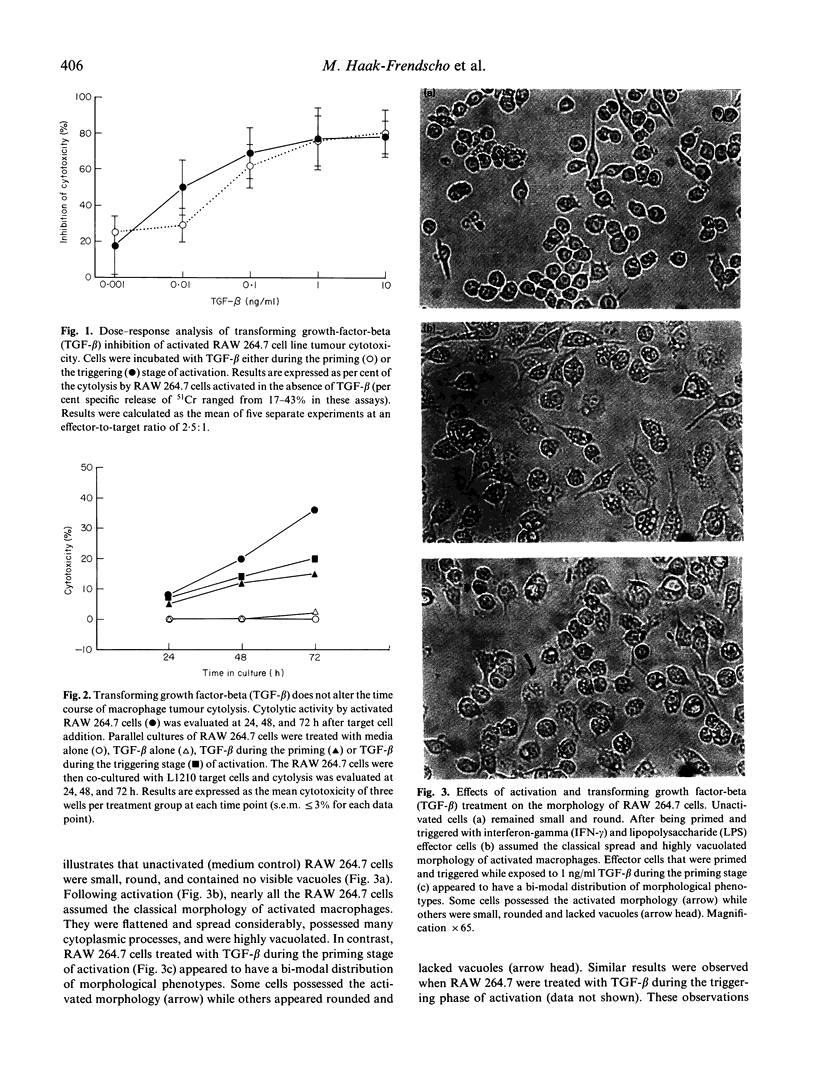
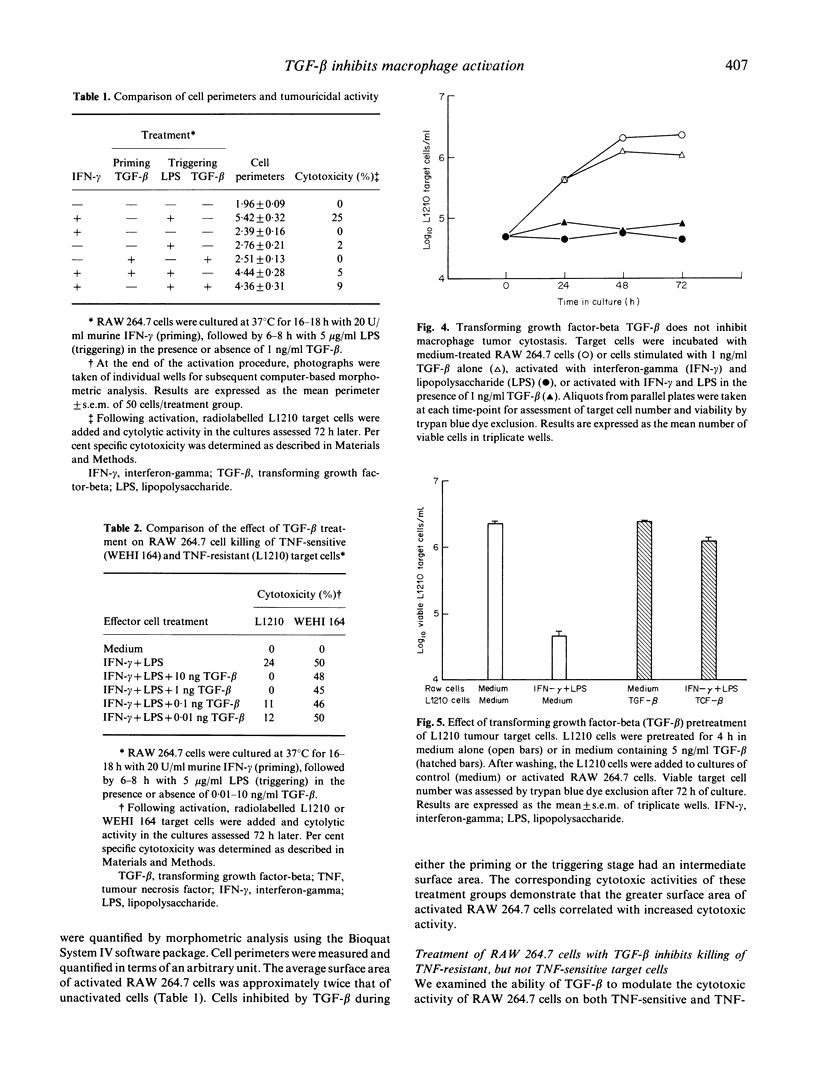



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzano M. A., Roberts A. B., Meyers C. A., Komoriya A., Lamb L. C., Smith J. M., Sporn M. B. Synergistic interaction of two classes of transforming growth factors from murine sarcoma cells. Cancer Res. 1982 Nov;42(11):4776–4778. [PubMed] [Google Scholar]
- Assoian R. K., Frolik C. A., Roberts A. B., Miller D. M., Sporn M. B. Transforming growth factor-beta controls receptor levels for epidermal growth factor in NRK fibroblasts. Cell. 1984 Jan;36(1):35–41. doi: 10.1016/0092-8674(84)90071-0. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Komoriya A., Meyers C. A., Miller D. M., Sporn M. B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983 Jun 10;258(11):7155–7160. [PubMed] [Google Scholar]
- Centrella M., Canalis E. Transforming and nontransforming growth factors are present in medium conditioned by fetal rat calvariae. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7335–7339. doi: 10.1073/pnas.82.21.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapes S. K. Formalin-fixed macrophages bind tumor targets similarly to viable macrophages. J Leukoc Biol. 1989 Apr;45(4):322–328. doi: 10.1002/jlb.45.4.322. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Ranges G. E., Palladino M. A., Jr Transforming growth factor-beta 1 (TGF-beta 1) and recombinant human tumor necrosis factor-alpha reciprocally regulate the generation of lymphokine-activated killer cell activity. Comparison between natural porcine platelet-derived TGF-beta 1 and TGF-beta 2, and recombinant human TGF-beta 1. J Immunol. 1988 Apr 1;140(7):2312–2316. [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Shalaby M. R., Lackides G. A., Lewis G. D., Shepard H. M., Palladino M. A., Jr Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J Exp Med. 1987 Aug 1;166(2):571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm E. A., Crump W. L., 3rd, Durett A., Hester J. P., Lagoo-Deenadalayan S., Owen-Schaub L. B. TGF-beta inhibits the in vitro induction of lymphokine-activated killing activity. Cancer Immunol Immunother. 1988;27(1):53–58. doi: 10.1007/BF00205758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert L. E., Paulnock D. M. Differential induction of activation markers in macrophage cell lines by interferon-gamma. Cell Immunol. 1989 May;120(2):401–418. doi: 10.1016/0008-8749(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Lambert L. E., Paulnock D. M. Modulation of macrophage function by gamma-irradiation. Acquisition of the primed cell intermediate stage of the macrophage tumoricidal activation pathway. J Immunol. 1987 Oct 15;139(8):2834–2841. [PubMed] [Google Scholar]
- Lee G., Ellingsworth L. R., Gillis S., Wall R., Kincade P. W. Beta transforming growth factors are potential regulators of B lymphopoiesis. J Exp Med. 1987 Nov 1;166(5):1290–1299. doi: 10.1084/jem.166.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino P. A., Adams D. O. The capacity of activated murine macrophages for augmented binding of neoplastic cells: analysis of induction by lymphokine containing MAF and kinetics of the reaction. J Immunol. 1982 Jun;128(6):2816–2823. [PubMed] [Google Scholar]
- Meltzer M. S. Macrophage activation for tumor cytotoxicity: characterization of priming and trigger signals during lymphokine activation. J Immunol. 1981 Jul;127(1):179–183. [PubMed] [Google Scholar]
- Mulé J. J., Schwarz S. L., Roberts A. B., Sporn M. B., Rosenberg S. A. Transforming growth factor-beta inhibits the in vitro generation of lymphokine-activated killer cells and cytotoxic T cells. Cancer Immunol Immunother. 1988;26(2):95–100. doi: 10.1007/BF00205600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W. Activation of mouse macrophages for tumor cell killing. I. Quantitative analysis of interactions between lymphokine and lipopolysaccharide. J Immunol. 1981 May;126(5):1863–1867. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Petit-Koskas E., Génot E., Lawrence D., Kolb J. P. Inhibition of the proliferative response of human B lymphocytes to B cell growth factor by transforming growth factor-beta. Eur J Immunol. 1988 Jan;18(1):111–116. doi: 10.1002/eji.1830180117. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Meyers C. A., Wideman J., Blacher R., Pan Y. C., Stein S., Lehrman S. R., Smith J. M., Lamb L. C. Purification and properties of a type beta transforming growth factor from bovine kidney. Biochemistry. 1983 Dec 6;22(25):5692–5698. doi: 10.1021/bi00294a002. [DOI] [PubMed] [Google Scholar]
- Rook A. H., Kehrl J. H., Wakefield L. M., Roberts A. B., Sporn M. B., Burlington D. B., Lane H. C., Fauci A. S. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986 May 15;136(10):3916–3920. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Macrophage activation for tumor cytotoxicity: development of macrophage cytotoxic activity requires completion of a sequence of short-lived intermediary reactions. J Immunol. 1978 Nov;121(5):2035–2042. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Strassmann G., Cole M. D., Newman W. Regulation of colony-stimulating factor 1-dependent macrophage precursor proliferation by type beta transforming growth factor. J Immunol. 1988 Apr 15;140(8):2645–2651. [PubMed] [Google Scholar]
- Takehara K., LeRoy E. C., Grotendorst G. R. TGF-beta inhibition of endothelial cell proliferation: alteration of EGF binding and EGF-induced growth-regulatory (competence) gene expression. Cell. 1987 May 8;49(3):415–422. doi: 10.1016/0092-8674(87)90294-7. [DOI] [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Riethmüller G. A rapid assay for cytotoxicity of unstimulated human monocytes. J Natl Cancer Inst. 1984 Jan;72(1):23–29. doi: 10.1093/jnci/72.1.23. [DOI] [PubMed] [Google Scholar]