Abstract
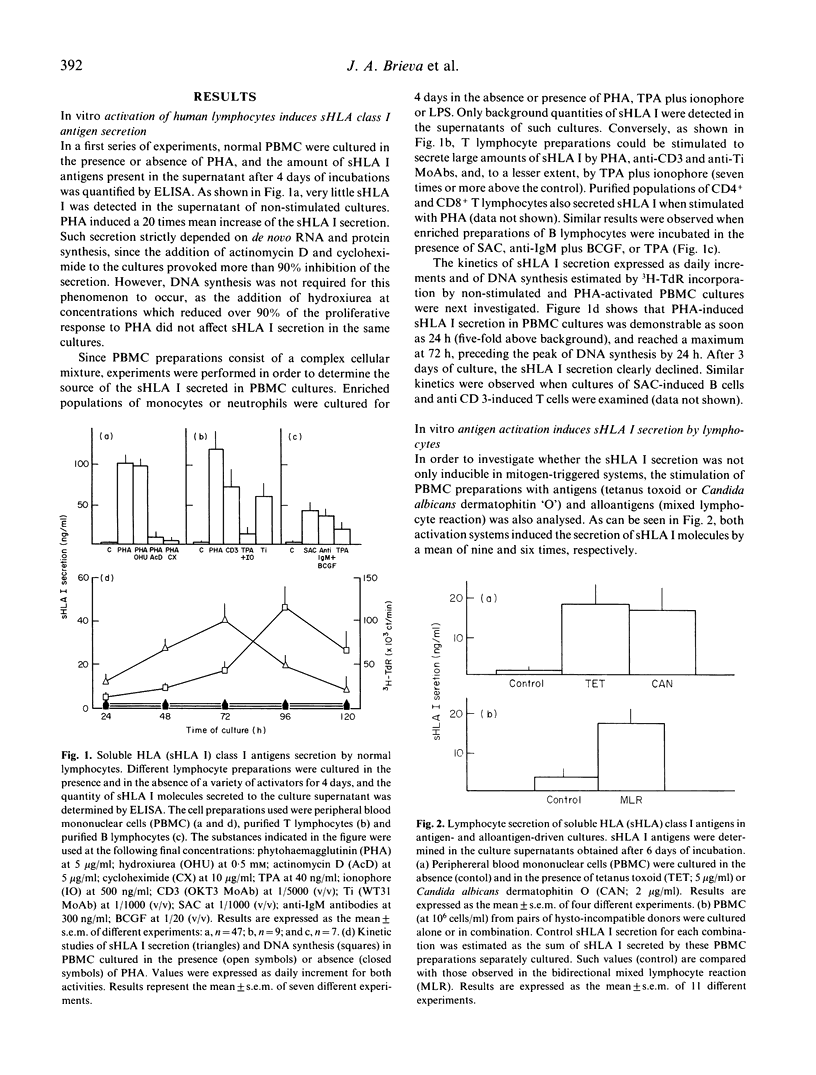
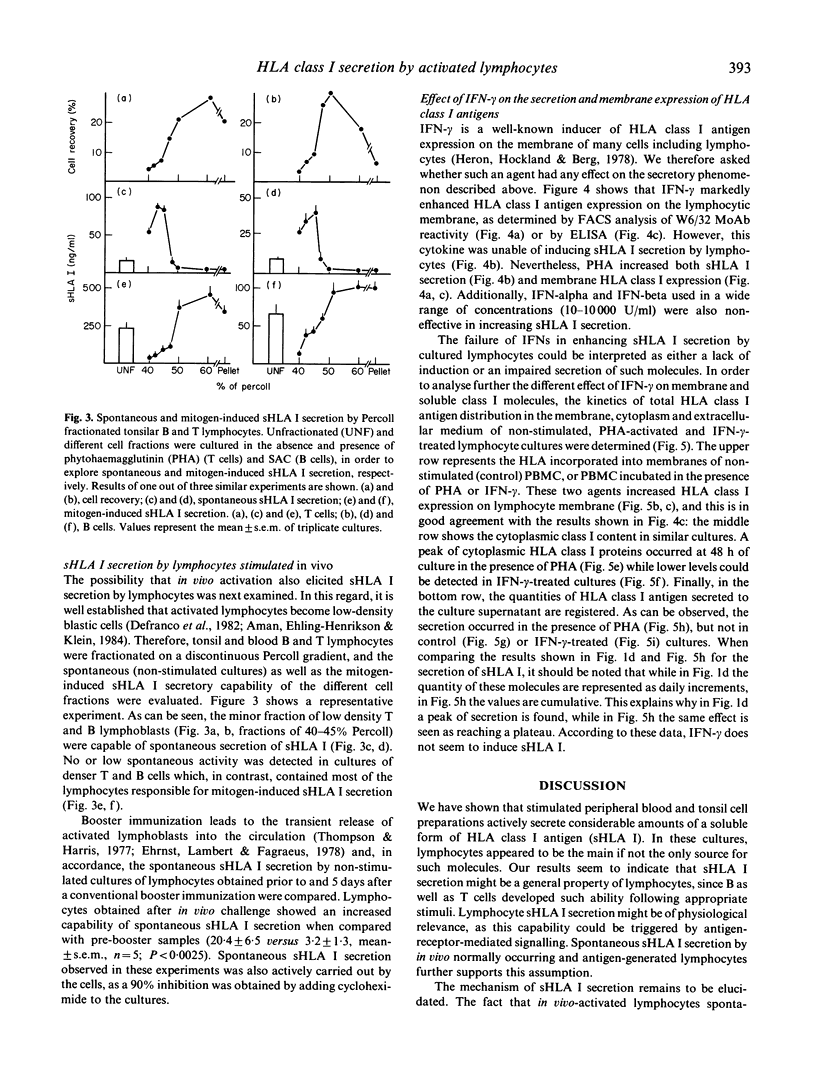
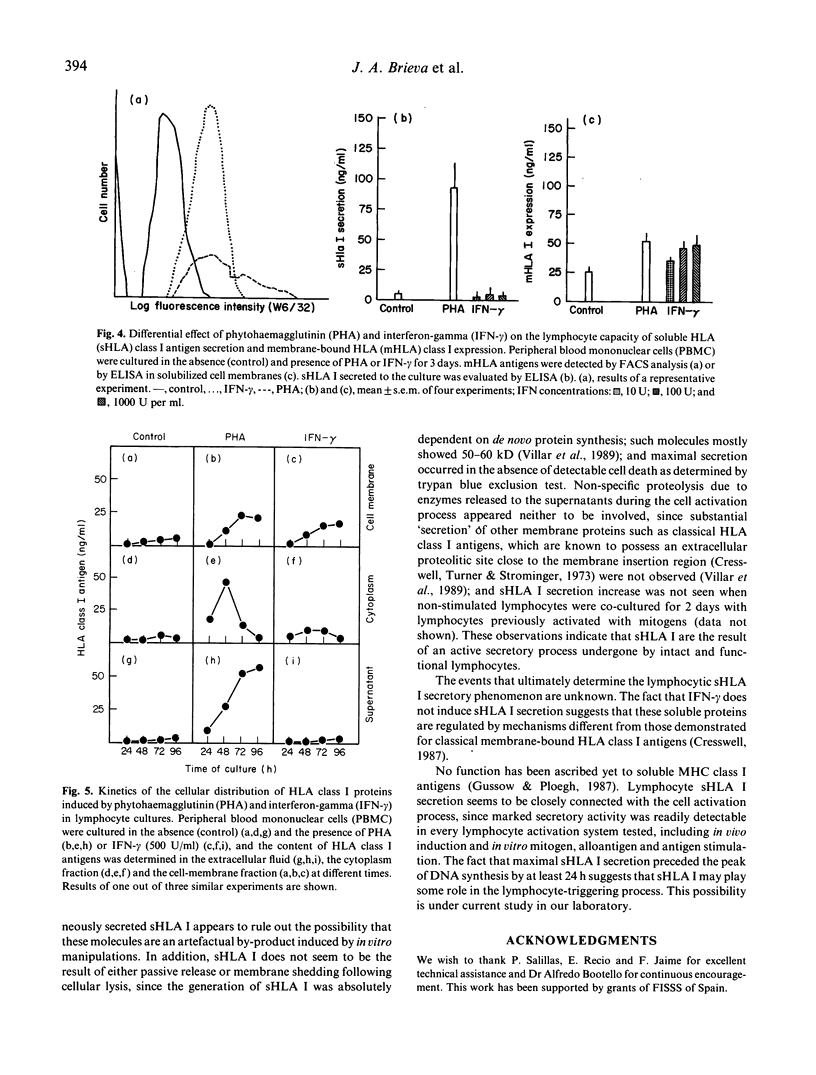
HLA class I antigens are thought to be integral membrane proteins. However, soluble forms of these molecules have been detected. Our laboratory has recently shown that the predominant form of these soluble proteins present in human serum, spleen tissue and culture supernatant of activated lymphocytes exhibits molecular weight and structure similar to classical HLA class I antigens, but lacks HLA A or B polymorphic determinants. In the present study, the secretion of such soluble proteins by lymphocytes has been further explored. Phytohaemagglutinin-stimulated normal lymphocytes secrete considerable quantities of soluble HLA (sHLA) class I proteins. This secretion seems to be a general property of lymphocytes, since activation of T as well as B cells by appropriate mitogens equally induce sHLA I secretion. Lymphocytes require RNA and protein synthesis, but not DNA synthesis, for the secretion to occur. Kinetic studies reveal that maximal sHLA I secretion precedes the peak of DNA synthesis by 24 h. In vitro stimulation with antigens or alloantigens also provokes sHLA I secretion. Moreover, this phenomenon has also been detected for in vivo-activated lymphocytes, as enhanced spontaneous sHLA I secretion was observed in cultures of low-density blastic B and T cells, and of blood lymphocytes obtained from normal subjects who had received a booster immunization 5 days earlier. Interferon-gamma (IFN-gamma) increases the expression of membrane-bound class I antigens but does not induce any sHLA I secretion, suggesting that both molecules are under different regulatory mechanisms. Our results indicate that human lymphocytes, upon stimulation, actively secrete considerable amounts of a soluble form of these biologically relevant proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aman P., Ehlin-Henriksson B., Klein G. Epstein-Barr virus susceptibility of normal human B lymphocyte populations. J Exp Med. 1984 Jan 1;159(1):208–220. doi: 10.1084/jem.159.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Brieva J. A., Targan S., Stevens R. H. NK and T cell subsets regulate antibody production by human in vivo antigen-induced lymphoblastoid B cells. J Immunol. 1984 Feb;132(2):611–615. [PubMed] [Google Scholar]
- Cresswell P. Regulation of HLA class I and class II antigen expression. Br Med Bull. 1987 Jan;43(1):66–80. doi: 10.1093/oxfordjournals.bmb.a072177. [DOI] [PubMed] [Google Scholar]
- Cresswell P., Turner M. J., Strominger J. L. Papain-solubilized HL-A antigens from cultured human lymphocytes contain two peptide fragments. Proc Natl Acad Sci U S A. 1973 May;70(5):1603–1607. doi: 10.1073/pnas.70.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbe L. M., Stam N. J., Neefjes J. J., Giphart M. J. Biochemical complexity of serum HLA class I molecules. Immunogenetics. 1988;27(3):203–210. doi: 10.1007/BF00346587. [DOI] [PubMed] [Google Scholar]
- Ehrnst A., Lambert B., Fagraeus A. DNA synthesis in subpopulations of blood mononuclear leucocytes in human subjects after vaccination against yellow fever. Scand J Immunol. 1978;8(4):339–346. doi: 10.1111/j.1365-3083.1978.tb00527.x. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Revilla Y., Bootello A., Gonzalez-Porqué P. Use of the ELISA to detect and quantify histocompatibility antigens and their subunits. J Immunol Methods. 1983 Dec 30;65(3):373–381. doi: 10.1016/0022-1759(83)90132-1. [DOI] [PubMed] [Google Scholar]
- Geraghty D. E., Koller B. H., Orr H. T. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron I., Hokland M., Berg K. Enhanced expression of beta2-microglobulin and HLA antigens on human lymphoid cells by interferon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6215–6219. doi: 10.1073/pnas.75.12.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Krangel M. S. Secretion of HLA-A and -B antigens via an alternative RNA splicing pathway. J Exp Med. 1986 May 1;163(5):1173–1190. doi: 10.1084/jem.163.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S. Two forms of HLA class I molecules in human plasma. Hum Immunol. 1987 Oct;20(2):155–165. doi: 10.1016/0198-8859(87)90029-2. [DOI] [PubMed] [Google Scholar]
- Kress M., Cosman D., Khoury G., Jay G. Secretion of a transplantation-related antigen. Cell. 1983 Aug;34(1):189–196. doi: 10.1016/0092-8674(83)90149-6. [DOI] [PubMed] [Google Scholar]
- Lew A. M., Maloy W. L., Coligan J. E. Characteristics of the expression of the murine soluble class I molecule (Q10). J Immunol. 1986 Jan;136(1):254–258. [PubMed] [Google Scholar]
- Paul P., Fauchet R., Boscher M. Y., Sayagh B., Masset M., Medrignac G., Dausset J., Cohen D. Isolation of a human major histocompatibility complex class I gene encoding a nonubiquitous molecule expressed on activated lymphocytes. Proc Natl Acad Sci U S A. 1987 May;84(9):2872–2876. doi: 10.1073/pnas.84.9.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M. A., Russo C., Allison J. P. HLA antigens in serum. Methods Enzymol. 1984;108:614–624. doi: 10.1016/s0076-6879(84)08122-2. [DOI] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Revilla Y., Ferreira A., Villar M. L., Bootello A., Gonzalez-Porqué P. Studies on the quaternary structure of class I major histocompatibility complex antigens. Effect of different agents on the interaction between subunits. J Biol Chem. 1986 May 15;261(14):6486–6491. [PubMed] [Google Scholar]
- Sen M. L., Garcia-Alonso A., Brieva J. A. Human B lymphocytes capable of spontaneous Ig production in short-term cultures: characterization in the circulation and lymphoid tissues. Cell Immunol. 1986 Mar;98(1):200–210. doi: 10.1016/0008-8749(86)90280-7. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Geraghty D. E., Koller B. H., Orr H. T., DeMars R. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. B., Brown R. E., Roser B. Class I transplantation antigens in solution in body fluids and in the urine. Individuality signals to the environment. J Exp Med. 1988 Jul 1;168(1):195–211. doi: 10.1084/jem.168.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. C., Fabre J. W. Identification in rat liver and serum of water-soluble class I MHC molecules possibly homologous to the murine Q10 gene product. J Exp Med. 1987 Jun 1;165(6):1595–1608. doi: 10.1084/jem.165.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Chorney M. J., Lawrance S. K., Pan J., Smith Z., Smith C. L., Weissman S. M. Structure, expression, and molecular mapping of a divergent member of the class I HLA gene family. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4224–4228. doi: 10.1073/pnas.84.12.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson P. D., Harris N. S. Detection of plaque-forming cells in the peripheral blood of actively immunized humans. J Immunol. 1977 Apr;118(4):1480–1482. [PubMed] [Google Scholar]
- Villar L. M., Roy G., Lázaro I., Alvarez-Cermeno J. C., González M., Brieva J. A., Del la Sen M. L., Leoro G., Bootello A., González-Porqué P. Detection of soluble class I molecules (non HLA-A or HLA-B) in serum, spleen membranes and lymphocytes in culture. Eur J Immunol. 1989 Oct;19(10):1835–1839. doi: 10.1002/eji.1830191012. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]


