Abstract
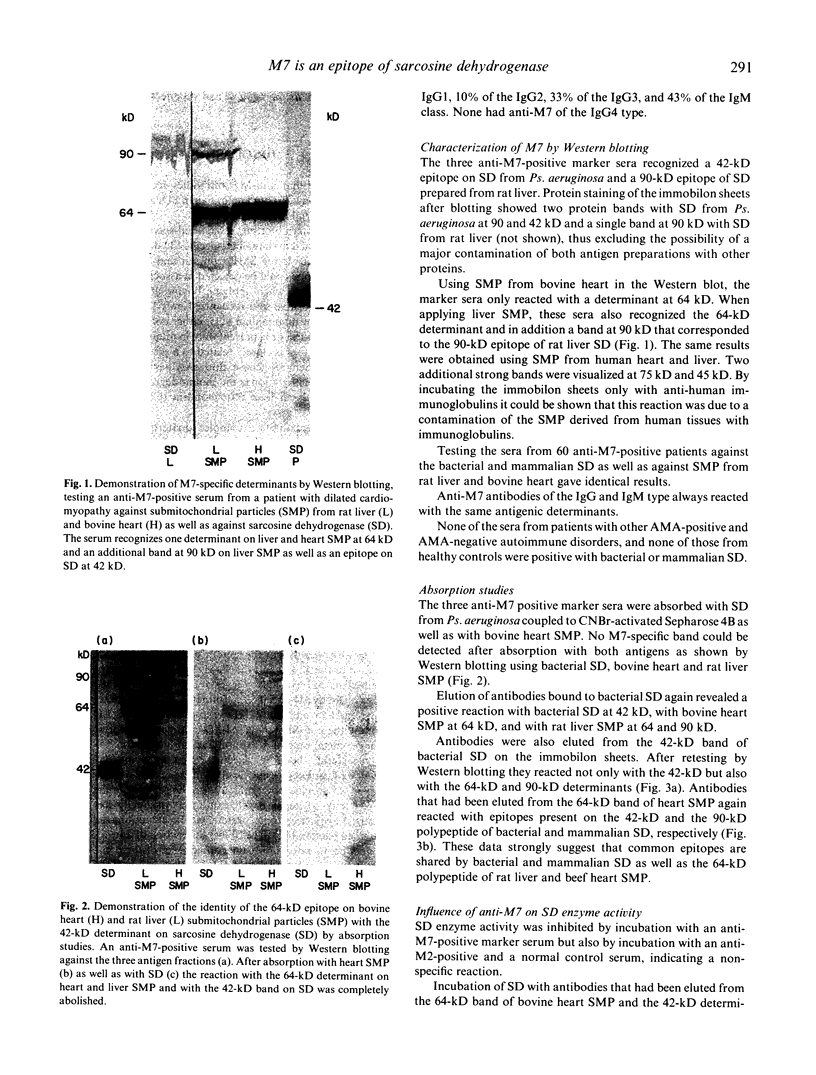
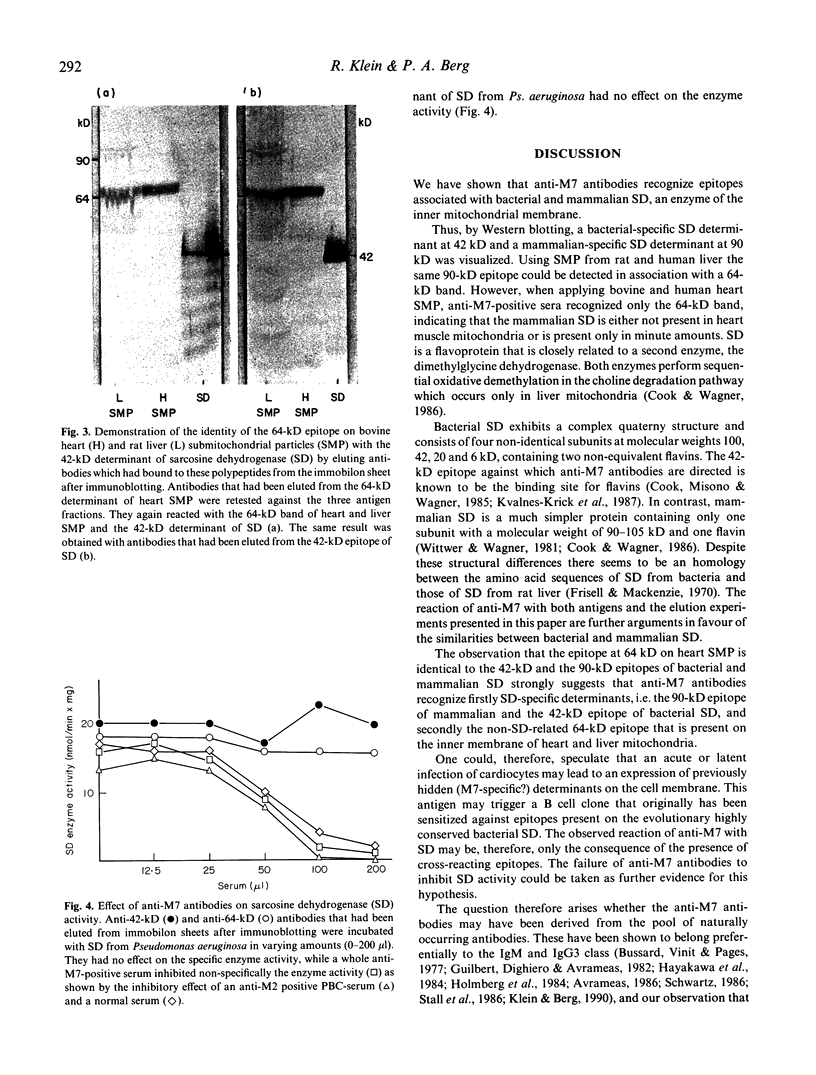
The anti-mitochondrial antibody (AMA) anti-M7 has been shown to occur exclusively in sera from patients with acute and chronic myocarditis. Applying different enzymes of the inner mitochondrial membrane to ELISA, anti-M7-positive sera reacted only with sarcosine dehydrogenase (SD) from Pseudomonas aeruginosa. Testing these sera in the Western blot against a commercially available SD as well as against SD prepared from rat liver mitochondria, a determinant at 42 kD and 90 kD, respectively, was visualized. Using submitochondrial particles (SMP) from bovine heart and rat liver another major determinant at 64 kD could be observed with both antigen fractions. Liver SMP also expressed the SD-related, 90-kD epitope. Sera from patients with other AMA-positive and AMA-negative autoimmune diseases were negative with these different determinants. The identity of the 64-kD epitope on heart and liver SMP as well as the 42-kD polypeptide of bacterial SD and the 90-kD epitope on mammalian SD was proven by absorption studies and by elution of antibodies from the antigen bound to the immobilon sheets after immunoblotting. The SD enzyme activity was not affected by anti-64-kD and anti-42-kD antibodies in vitro. It is concluded that anti-M7 antibodies may be stimulated by an antigen expressed on cardiocytes during an infection which shares epitopes with SD, an evolutionary highly conserved protein. SD-sensitized B cell clones could therefore be triggered by the M7-antigen which shows homology to SD.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autore C., Fiorito S., Pelliccia A., Caselli G., Fragola P. V., Picelli A., Maccari A. M., Pocobelli D., Cannata D., Sangiorgi M. Antimitochondrial autoantibodies in myocardial hypertrophy: comparison between hypertrophic cardiomyopathy, hypertensive heart disease, and athlete's heart. Am Heart J. 1988 Aug;116(2 Pt 1):496–500. doi: 10.1016/0002-8703(88)90623-0. [DOI] [PubMed] [Google Scholar]
- Berg P. A., Doniach D., Roitt I. M. Mitochondrial antibodies in primary biliary cirrhosis. I. Localization of the antigen to mitochondrial membranes. J Exp Med. 1967 Aug 1;126(2):277–290. doi: 10.1084/jem.126.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg P. A., Klein R. Heterogeneity of antimitochondrial antibodies. Semin Liver Dis. 1989 May;9(2):103–116. doi: 10.1055/s-2008-1040501. [DOI] [PubMed] [Google Scholar]
- Bussard A. E., Vinit M. A., Pages J. M. Immunochemical characterization of the auto antibodies produced by mouse peritoneal cells in culture. Immunochemistry. 1977 Jan;14(1):1–9. doi: 10.1016/0019-2791(77)90326-3. [DOI] [PubMed] [Google Scholar]
- Cook R. J., Wagner C. Dimethylglycine dehydrogenase and sarcosine dehydrogenase: mitochondrial folate-binding proteins from rat liver. Methods Enzymol. 1986;122:255–260. doi: 10.1016/0076-6879(86)22179-5. [DOI] [PubMed] [Google Scholar]
- Guilbert B., Dighiero G., Avrameas S. Naturally occurring antibodies against nine common antigens in human sera. I. Detection, isolation and characterization. J Immunol. 1982 Jun;128(6):2779–2787. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg D., Forsgren S., Ivars F., Coutinho A. Reactions among IgM antibodies derived from normal, neonatal mice. Eur J Immunol. 1984 May;14(5):435–441. doi: 10.1002/eji.1830140510. [DOI] [PubMed] [Google Scholar]
- Klein R., Maisch B., Kochsiek K., Berg P. A. Demonstration of organ specific antibodies against heart mitochondria (anti-M7) in sera from patients with some forms of heart diseases. Clin Exp Immunol. 1984 Nov;58(2):283–292. [PMC free article] [PubMed] [Google Scholar]
- Kvalnes-Krick K., Jorns M. S. Interaction of tetrahydrofolate and other folate derivatives with bacterial sarcosine oxidase. Biochemistry. 1987 Nov 17;26(23):7391–7395. doi: 10.1021/bi00397a029. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maisch B., Deeg P., Liebau G., Kochsiek K. Diagnostic relevance of humoral and cytotoxic immune reactions in primary and secondary dilated cardiomyopathy. Am J Cardiol. 1983 Nov 1;52(8):1072–1078. doi: 10.1016/0002-9149(83)90535-0. [DOI] [PubMed] [Google Scholar]
- Neu N., Beisel K. W., Traystman M. D., Rose N. R., Craig S. W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to Coxsackievirus B3-induced myocarditis. J Immunol. 1987 Apr 15;138(8):2488–2492. [PubMed] [Google Scholar]
- Rose N. R., Herskowitz A., Neumann D. A., Neu N. Autoimmune myocarditis: a paradigm of post-infection autoimmune disease. Immunol Today. 1988 Apr;9(4):117–120. doi: 10.1016/0167-5699(88)91282-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer A. J., Wagner C. Identification of the folate-binding proteins of rat liver mitochondria as dimethylglycine dehydrogenase and sarcosine dehydrogenase. Purification and folate-binding characteristics. J Biol Chem. 1981 Apr 25;256(8):4102–4108. [PubMed] [Google Scholar]





