Abstract
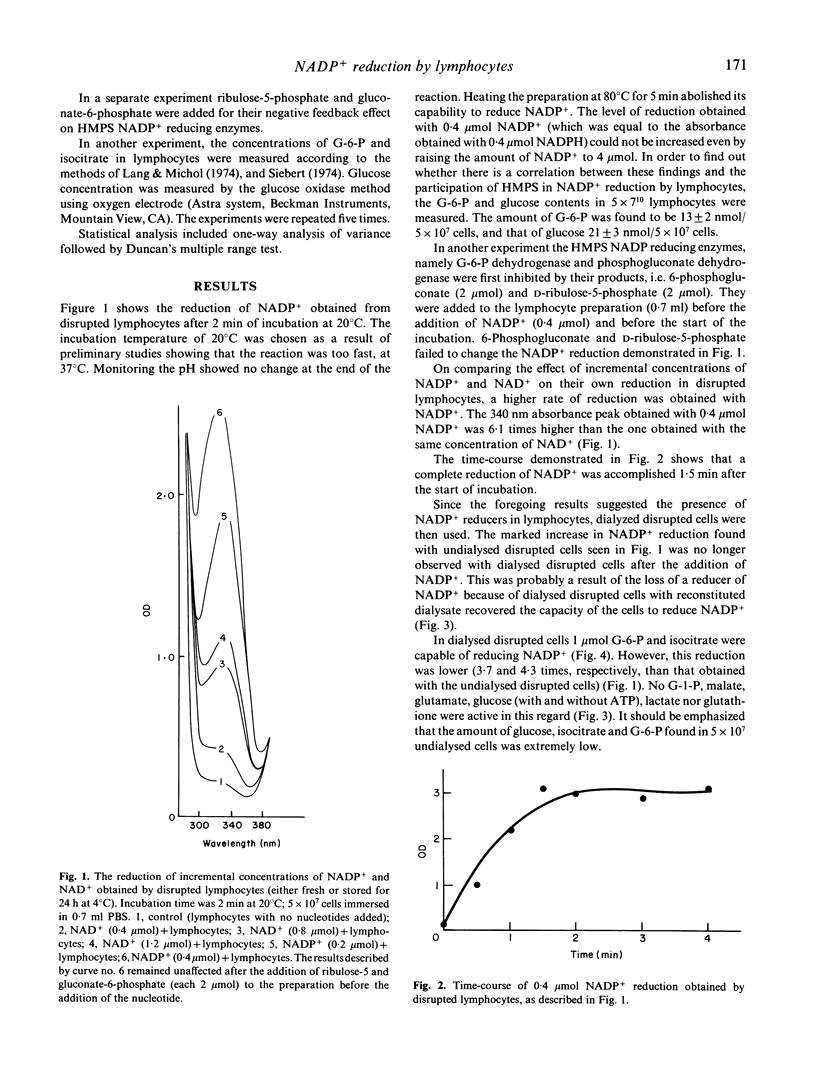
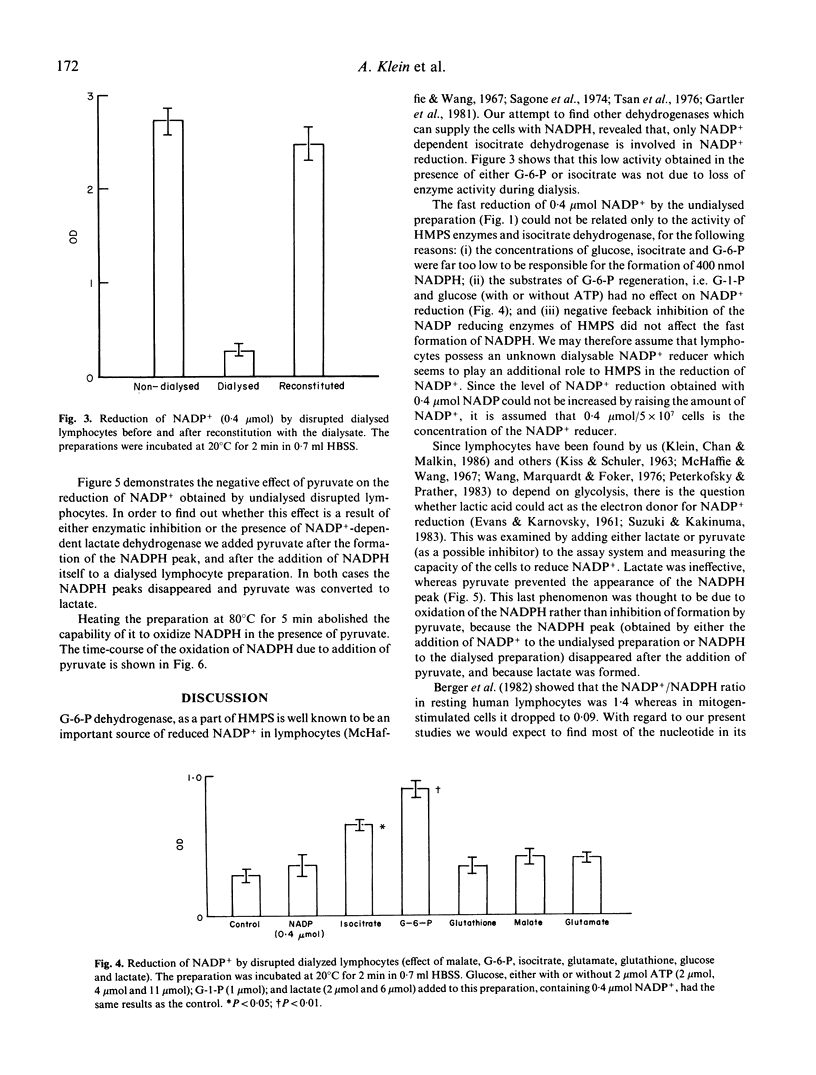
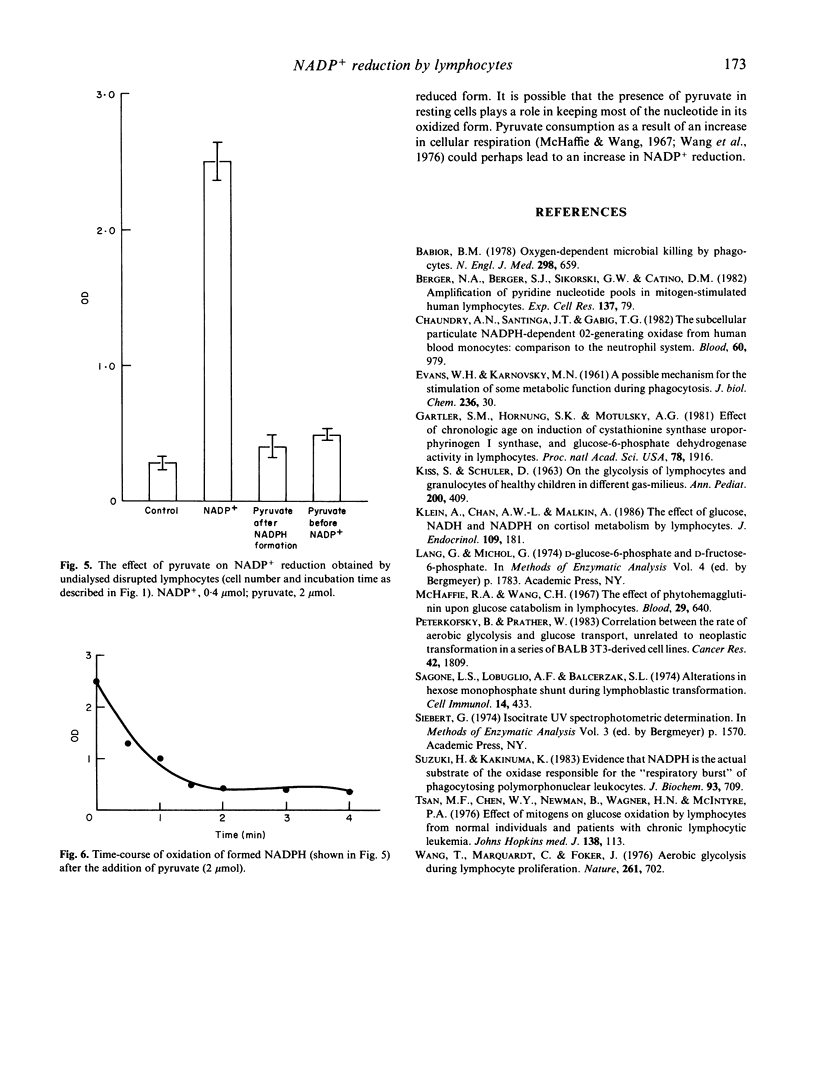
The hexose monophosphate shunt (HMPS) is known to be responsible for the reduction of NADP+ by lymphocytes. We tried to find other enzymatic systems that might provide the lymphocytes with NADPH. By measuring the absorbance at 340 nm we noted that the addition of NADP+ to a preparation of disrupted lymphocytes resulted in the formation of NADPH at a rate of 4 nmol/10(6) cells per min. This phenomenon could not be changed by negative feedback inhibition of HMPS, and could not be attributed to the low concentration of glucose, glucose-6-phosphate (G-6-P) and isocitrate found in the cell preparation (NADP(+)-dependent isocitrate dehydrogenase in addition to HMPS NADP+ reducing enzymes was found to be present in lymphocytes). Because of the activity of a NADP(+)-dependent lactate dehydrogenase, pyruvate oxidized the NADPH as it was being formed. Here we demonstrate the presence of an unknown NADP+ reducer in lymphocytes which seems to play an additional role to HMPS in NADP+ reduction by lymphocytes. NADP(+)-dependent lactate dehydrogenase may play a role in regulating the NADP+/NADPH ratio.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Berger N. A., Berger S. J., Sikorski G. W., Catino D. M. Amplification of pyridine nucleotide pools in mitogen-stimulated human lymphocytes. Exp Cell Res. 1982 Jan;137(1):79–88. doi: 10.1016/0014-4827(82)90010-6. [DOI] [PubMed] [Google Scholar]
- Chaudhry A. N., Santinga J. T., Gabig T. G. The subcellular particulate NADPH-dependent O2.(-)-generating oxidase from human blood monocytes: comparison to the neutrophil system. Blood. 1982 Oct;60(4):979–983. [PubMed] [Google Scholar]
- Gartler S. M., Hornung S. K., Motulsky A. G. Effect of chronologic age on induction of cystathionine synthase, uroporphyrinogen I synthase, and glucose-6-phosphate dehydrogenase activities in lymphocytes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1916–1919. doi: 10.1073/pnas.78.3.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISS S., SCHULER D. On the glycolysis of lymphocytes and granulocytes of health children in different gas-milieus. Ann Paediatr. 1963;200:409–416. [PubMed] [Google Scholar]
- Klein A., Chan A. W., Malkin A. Effect of glucose, NADH and NADPH on cortisol metabolism by mononuclear cells. J Endocrinol. 1986 May;109(2):181–185. doi: 10.1677/joe.0.1090181. [DOI] [PubMed] [Google Scholar]
- MacHaffie R. A., Wang C. H. The effect of phytohemagglutinin upon glucose catabolism in lymphocytes. Blood. 1967 Apr;29(4 Suppl):640–646. [PubMed] [Google Scholar]
- Peterkofsky B., Prather W. Correlation between the rates of aerobic glycolysis and glucose transport, unrelated to neoplastic transformation, in a series of BALB 3T3-derived cell lines. Cancer Res. 1982 May;42(5):1809–1816. [PubMed] [Google Scholar]
- Suzuki H., Kakinuma K. Evidence that NADPH is the actual substrate of the oxidase responsible for the "respiratory burst" of phagocytosing polymorphonuclear leukocytes. J Biochem. 1983 Mar;93(3):709–715. doi: 10.1093/jb/93.3.709. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Chen W. Y., Newman B., Wagner H. N., Jr, McIntyre P. A. Effects of mitogens on glucose oxidation by lymphocytes from normal individuals and patients with chronic lymphocytic leukemia. Johns Hopkins Med J. 1976 Apr;138(4):113–118. [PubMed] [Google Scholar]
- Wang T., Marquardt C., Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976 Jun 24;261(5562):702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]


