Abstract
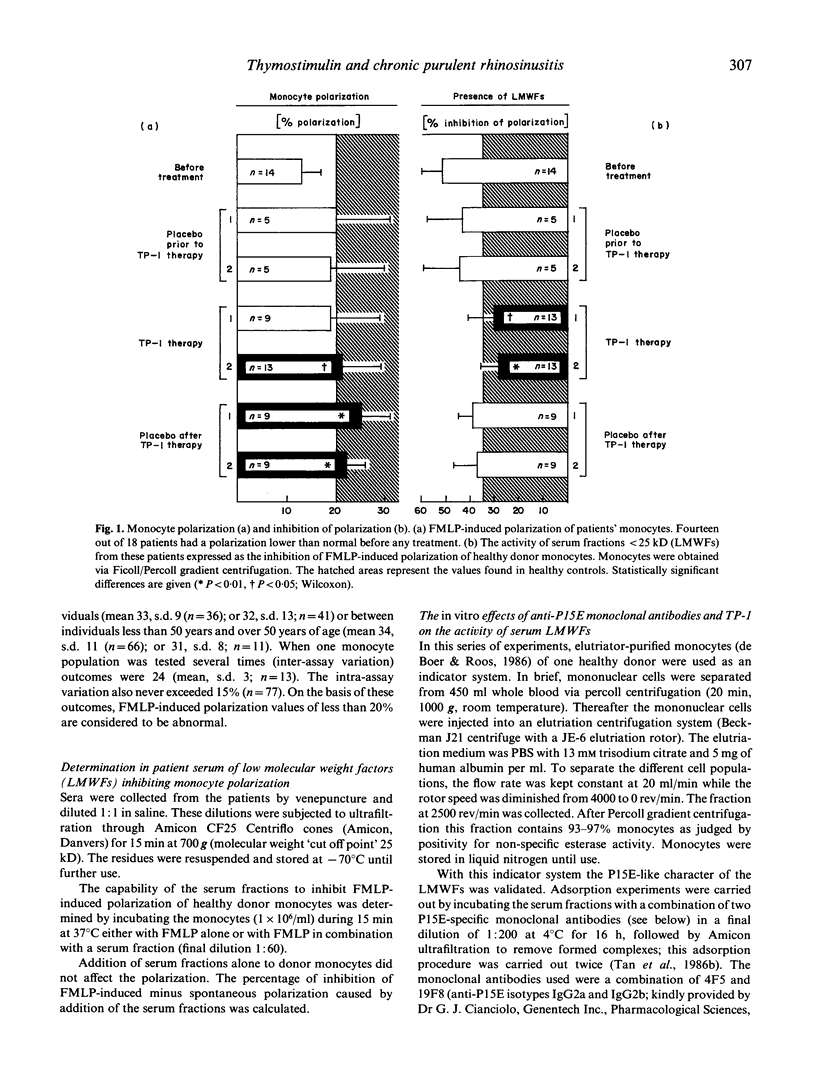
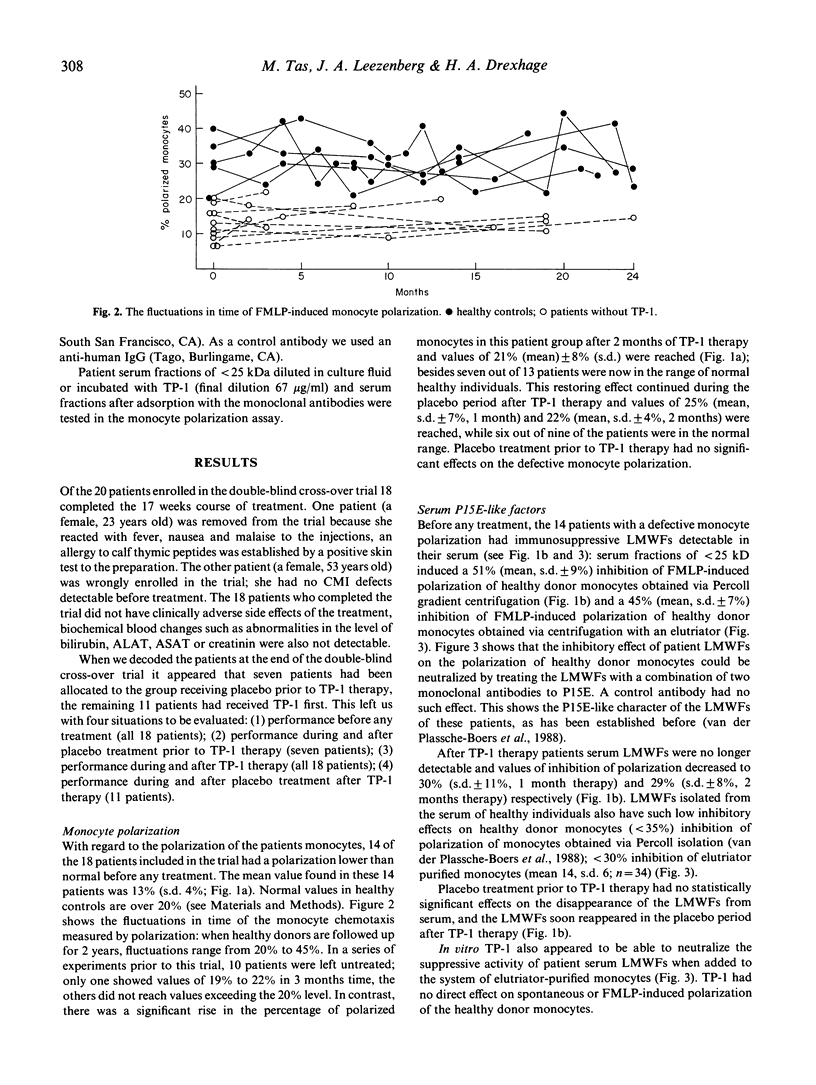
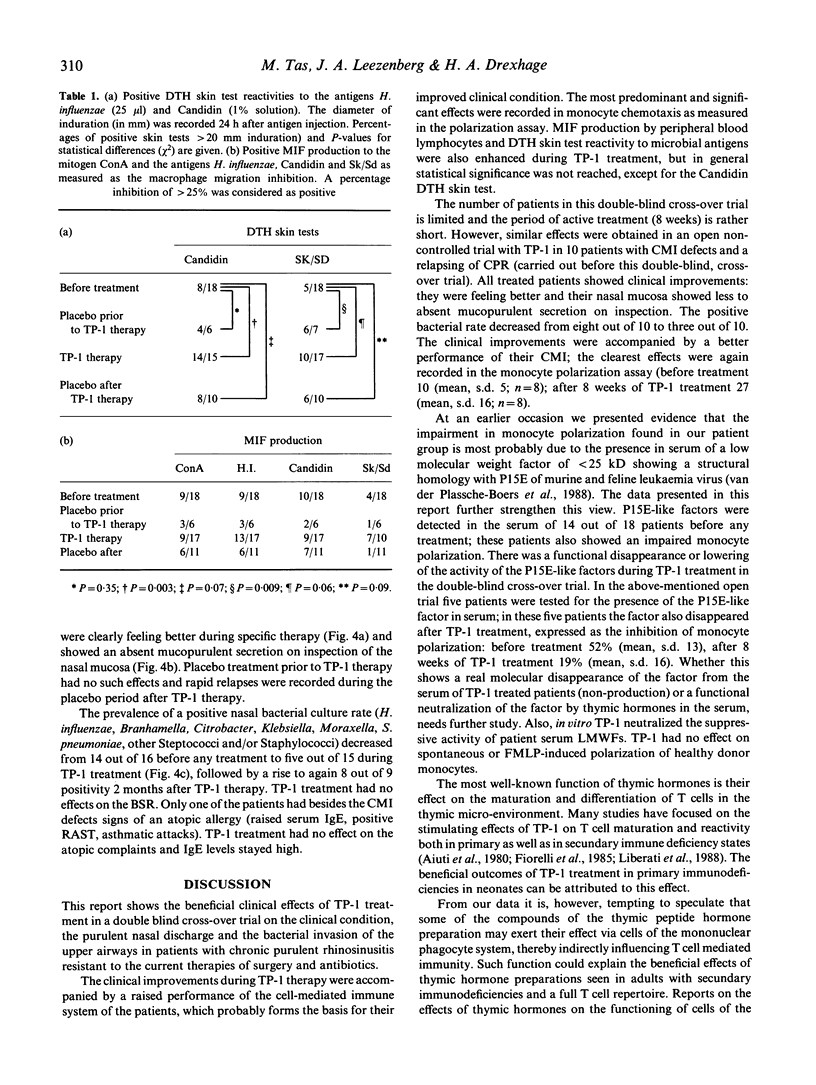
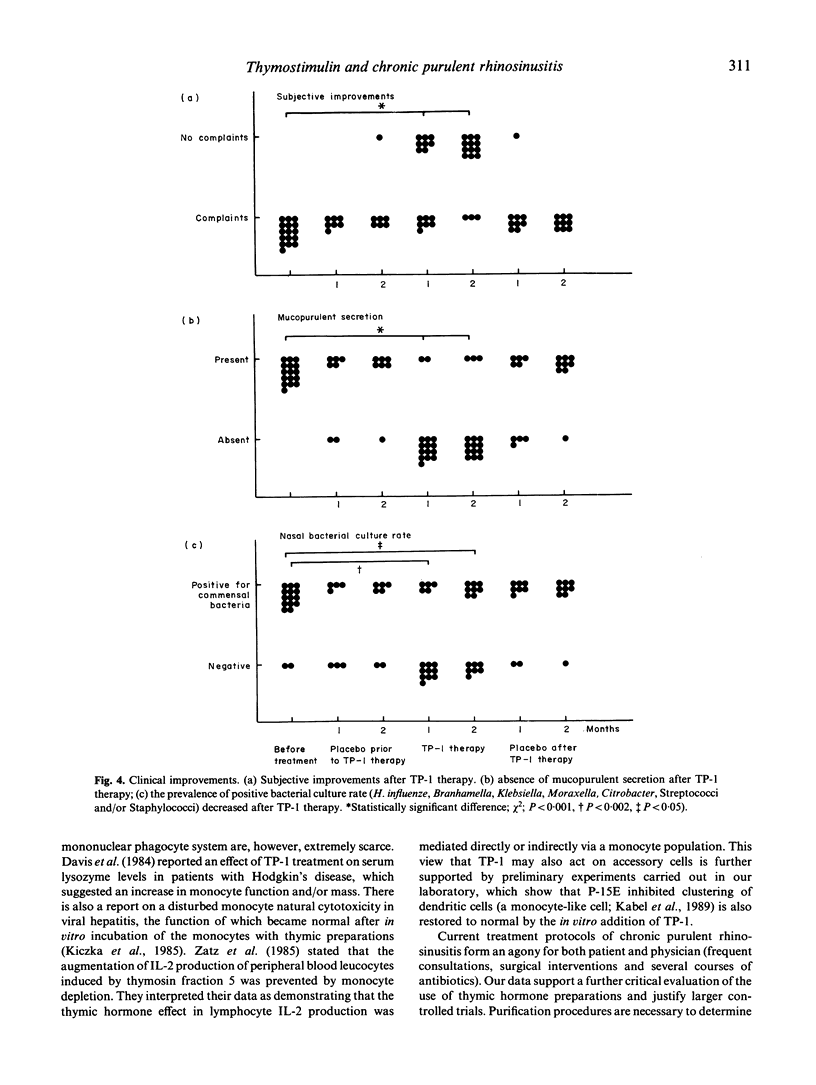
Twenty patients with chronic purulent rhinosinusitis were treated with TP-1 (Serono; 1 mg/kg body weight), in a double-blind cross-over trial. TP-1 was administered by daily i.m. injections for the first 14 days followed by two injections/week for 6 further weeks. The patients were immunologically special in that they had defects in their cell-mediated immune system. Fourteen showed a decreased chemotactic responsiveness of their peripheral blood monocytes as measured in the polarization assay. This defective function can probably be ascribed to the presence in serum of low molecular weight factors (LMWFs; less than 25 kD). As reported earlier, this factor shows a structural homology to the envelope protein of murine and feline leukaemia virus (P15E). Thirteen patients showed a defective delayed-type hypersensitivity (DTH) skin test reactivity towards candidin and/or streptokinase-streptodornase (Sk/Sd) antigen, 14 had a defective MIF production from their peripheral blood lymphocytes towards candidin, Sk/Sd and/or Haemophilus influenzae antigen. Eighteen patients completed the TP-1 trial and showed clinical improvements: 12 out of 15 were feeling better during TP-1 therapy and the nasal mucosa showed on inspection absent mucopurulent secretion in 13 patients. Positive bacterial culture rates for the nose decreased from 14 out of 16 to five out of 15. Placebo treatment had no significant effects. The clinical improvements were accompanied by a better performance of the cell-mediated immune system; the most significant effects were recorded in the monocyte polarization assay. The suppressive P15E-like LMWFs in serum clearly decreased during TP-1 treatment. In vitro TP-1 neutralized the immunosuppressive effect of the LMWFs. The restoring effects of TP-1 on monocyte polarization and its neutralizing activity of P15E-like LMWFs could explain the beneficial effects of thymic hormone treatment reported in adults with clinical signs of immunodeficiency in the presence of a full T cell repertoire.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balm F. J., von Blomberg-van deFlier B. M., Drexhage H. A., de Haan-Meulman M., Snow G. B. Mononuclear phagocyte function in head and neck cancer: depression of murine macrophage accumulation by low molecular weight factors derived from head and neck carcinomas. Laryngoscope. 1984 Feb;94(2 Pt 1):223–227. doi: 10.1288/00005537-198402000-00016. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Matthews T. J., Bolognesi D. P., Snyderman R. Macrophage accumulation in mice is inhibited by low molecular weight products from murine leukemia viruses. J Immunol. 1980 Jun;124(6):2900–2905. [PubMed] [Google Scholar]
- Cianciolo G. J., Phipps D., Snyderman R. Human malignant and mitogen-transformed cells contain retroviral P15E-related antigen. J Exp Med. 1984 Mar 1;159(3):964–969. doi: 10.1084/jem.159.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo G. J., Snyderman R. Monocyte responsiveness to chemotactic stimuli is a property of a subpopulation of cells that can respond to multiple chemoattractants. J Clin Invest. 1981 Jan;67(1):60–68. doi: 10.1172/JCI110033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo G., Hunter J., Silva J., Haskill J. S., Snyderman R. Inhibitors of monocyte responses to chemotaxins are present in human cancerous effusions and react with monoclonal antibodies to the P15(E) structural protein of retroviruses. J Clin Invest. 1981 Oct;68(4):831–844. doi: 10.1172/JCI110338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexhage H. A., van de Plassche E. M., Kokjé M., Leezenberg H. A. Abnormalities in cell-mediated immune functions to Haemophilus influenzae chronic purulent infections of the upper respiratory tract. Clin Immunol Immunopathol. 1983 Aug;28(2):218–228. doi: 10.1016/0090-1229(83)90156-3. [DOI] [PubMed] [Google Scholar]
- Furukawa C. T., Altman L. C. Defective monocyte and polymorphonuclear leukocyte chemotaxis in atopic disease. J Allergy Clin Immunol. 1978 May;61(5):288–293. doi: 10.1016/0091-6749(78)90049-0. [DOI] [PubMed] [Google Scholar]
- Iwata T., Incefy G. S., Cunningham-Rundles S., Cunningham-Rundles C., Smithwick E., Geller N., O'Reilly R., Good R. A. Circulating thymic hormone activity in patients with primary and secondary immunodeficiency diseases. Am J Med. 1981 Sep;71(3):385–394. doi: 10.1016/0002-9343(81)90164-9. [DOI] [PubMed] [Google Scholar]
- Iwata T., Incefy G. S., Good R. A., Cunningham-Rundles S., Dardenne M., Kapoor N., Kirkpatrick D., O'Reilly R. J. Circulating thymic hormone levels in severe combined immunodeficiency. Clin Exp Immunol. 1983 Jul;53(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Kabel P. J., de Haan-Meulman M., Voorbij H. A., Kleingeld M., Knol E. F., Drexhage H. A. Accessory cells with a morphology and marker pattern of dendritic cells can be obtained from elutriator-purified blood monocyte fractions. An enhancing effect of metrizamide in this differentiation. Immunobiology. 1989 Oct;179(4-5):395–341. doi: 10.1016/S0171-2985(89)80044-0. [DOI] [PubMed] [Google Scholar]
- Kiczka W., Szkaradkiewicz A., Flieger J. Studies on natural cytotoxicity of human monocytes in viral hepatitis. Immunol Lett. 1985;9(2-3):87–91. doi: 10.1016/0165-2478(85)90016-1. [DOI] [PubMed] [Google Scholar]
- Lewis V., Twomey J. J., Goldstein G., O'Reilly R., Smithwick E., Pahwa R., Pahwa S., Good R. A., Schulte-Wisserman H., Horowitz S. Circulating thymic-hormone activity in congenital immunodeficiency. Lancet. 1977 Sep 3;2(8036):471–475. doi: 10.1016/s0140-6736(77)91601-4. [DOI] [PubMed] [Google Scholar]
- Liberati A. M., Ballatori E., Fizzotti M., Schippa M., Cini L., Cinieri S., Proietti M. G., Di Marzio R., Senatore M., Grignani F. A randomized trial to evaluate the immunorestorative properties of thymostimulin in patients with Hodgkin's disease in complete remission. Cancer Immunol Immunother. 1988;26(1):87–93. doi: 10.1007/BF00199853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Hsu C. H., Liu K. C., Chen C. L., Hsu H. C. Serial immunologic and histopathologic studies in the treatment of necrotizing fasciitis with combined immunodeficiency by a bovine thymic extract (thymostimulin). J Pediatr Surg. 1986 Nov;21(11):1000–1004. doi: 10.1016/s0022-3468(86)80124-5. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Hsu H. C., Chen C. L., Shen E. Y. Treatment of combined immunodeficiency with thymic extract (Thymostimulin). Ann Allergy. 1987 May;58(5):379–384. [PubMed] [Google Scholar]
- Lin C. Y., Hsu H. C., Hsieh H. C. Treatment of progressive Bacillus Calmette-Guérin infection in an immunodeficient infant with a specific bovine thymic extract (thymostimulin). Pediatr Infect Dis. 1985 Jul-Aug;4(4):402–405. doi: 10.1097/00006454-198507000-00015. [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Kuo Y. C., Lin C. C., Ou B. R. Enhancement of interleukin-2 and gamma-interferon production in vitro on cord blood lymphocytes and in vivo on primary cellular immunodeficiency patients with thymic extract (thymostimulin). J Clin Immunol. 1988 Mar;8(2):103–107. doi: 10.1007/BF00917897. [DOI] [PubMed] [Google Scholar]
- Mullink H., Von Blomberg M., Wilders M. M., Drexhage H. A., Alons C. L. A simple cytochemical method for distinguishing EAC rosettes formed by lymphocytes and monocytes. J Immunol Methods. 1979;29(2):133–137. doi: 10.1016/0022-1759(79)90062-0. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Johnsson A., Wärmegård B., Seljelid R. Separation of human monocytes on density gradients of Percoll. J Immunol Methods. 1980;33(3):221–229. doi: 10.1016/0022-1759(80)90209-4. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Snyderman R. Depression of macrophage function by a factor produced by neoplasms: a merchanism for abrogation of immune surveillance. J Immunol. 1976 Oct;117(4):1243–1249. [PubMed] [Google Scholar]
- Tan I. B., Drexhage H. A., Mullink R., Hensen-Logmans S., De Haan-Meulman M., Snow G. B., Balm A. J. Immunohistochemical detection of retroviral-P15E-related material in carcinomas of the head and neck. Otolaryngol Head Neck Surg. 1987 Mar;96(3):251–255. doi: 10.1177/019459988709600304. [DOI] [PubMed] [Google Scholar]
- Tan I. B., Drexhage H. A., Scheper R. J., von Blomberg-van de Flier B. M., de Haan-Meulman M., Snow G. B., Balm F. J. Immunosuppressive retroviral P15E-related factors in head and neck carcinomas. Arch Otolaryngol Head Neck Surg. 1986 Sep;112(9):942–945. doi: 10.1001/archotol.1986.03780090038006. [DOI] [PubMed] [Google Scholar]
- Tas M., Drexhage H. A., Goudsmit J. A monocyte chemotaxis inhibiting factor in serum of HIV infected men shares epitopes with the HIV transmembrane protein gp41. Clin Exp Immunol. 1988 Jan;71(1):13–18. [PMC free article] [PubMed] [Google Scholar]
- Thurman G. B., Stull H. B., Miller P. J., Stevenson H. C., Oldham R. K. Utilization of purified human monocytes in the agarose droplet assay for measuring migration inhibitory factors. J Immunol Methods. 1983 Dec 16;65(1-2):41–53. doi: 10.1016/0022-1759(83)90302-2. [DOI] [PubMed] [Google Scholar]
- Van de Plassche-Boers E. M., Drexhage H. A., Kokjé-Kleingeld M. The use of somatic antigen of Haemophilus influenzae for the monitoring of T cell-mediated skin test reactivity in man. J Immunol Methods. 1985 Nov 7;83(2):353–361. doi: 10.1016/0022-1759(85)90257-1. [DOI] [PubMed] [Google Scholar]
- Zatz M. M., Skotnicki A., Bailey J. M., Oliver J. H., Goldstein A. L. Mechanism of action of thymosin. II. Effects of aspirin and thymosin on enhancement of IL-2 production. Immunopharmacology. 1985 Jun;9(3):189–198. doi: 10.1016/0162-3109(85)90015-3. [DOI] [PubMed] [Google Scholar]
- de Boer M., Roos D. Metabolic comparison between basophils and other leukocytes from human blood. J Immunol. 1986 May 1;136(9):3447–3454. [PubMed] [Google Scholar]
- van de Plassche-Boers E. M., Drexhage H. A., Kokjé-Kleingeld M., Leezenberg H. A. Parameters of T cell mediated immunity to commensal micro-organisms in patients with chronic purulent rhinosinusitis: a comparison between delayed type hypersensitivity skin test, lymphocyte transformation test and macrophage migration inhibition factor assay. Clin Exp Immunol. 1986 Dec;66(3):516–524. [PMC free article] [PubMed] [Google Scholar]
- van de Plassche-Boers E. M., Tas M., de Haan-Meulman M., Kleingeld M., Drexhage H. A. Abnormal monocyte chemotaxis in patients with chronic purulent rhinosinusitis: an effect of retroviral p15E-related factors in serum. Clin Exp Immunol. 1988 Sep;73(3):348–354. [PMC free article] [PubMed] [Google Scholar]
- van der Baan S., Veerman A. J., Heidendahl G. A., den Hollander W., Feenstra L. Primary ciliary dyskinesia and nasal mucociliary clearance. Respiration. 1987;52(1):69–75. doi: 10.1159/000195306. [DOI] [PubMed] [Google Scholar]


