Abstract
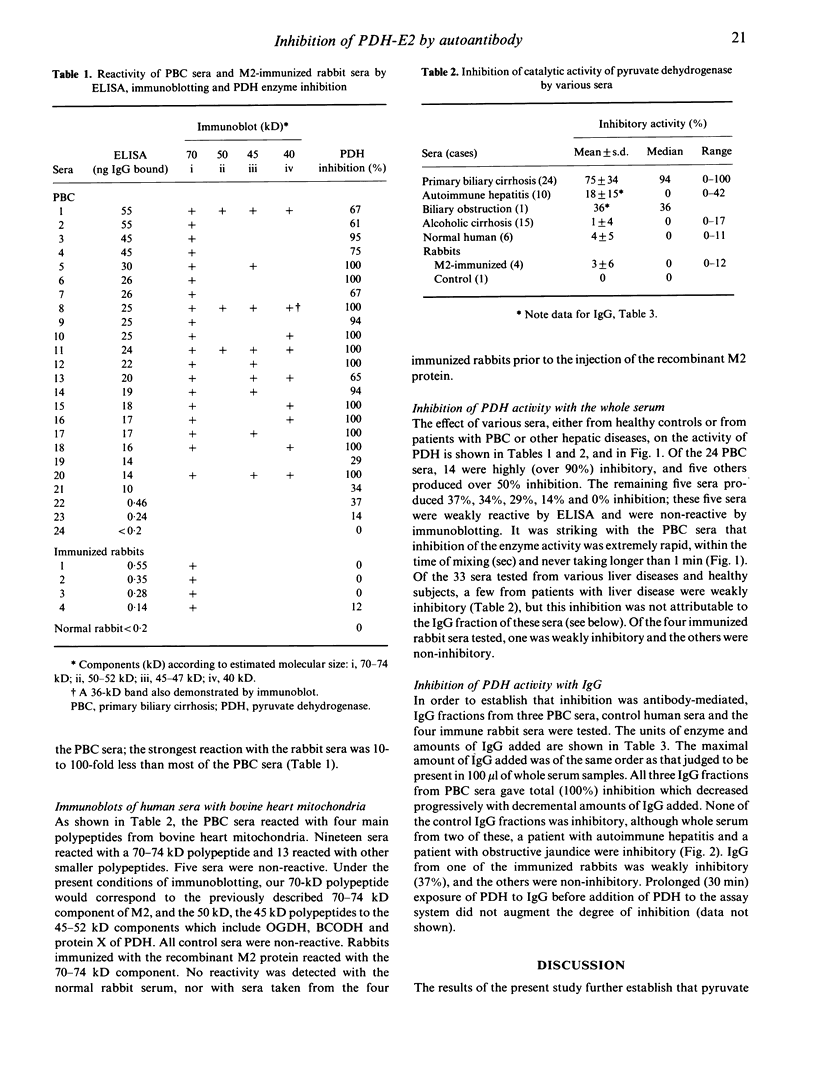
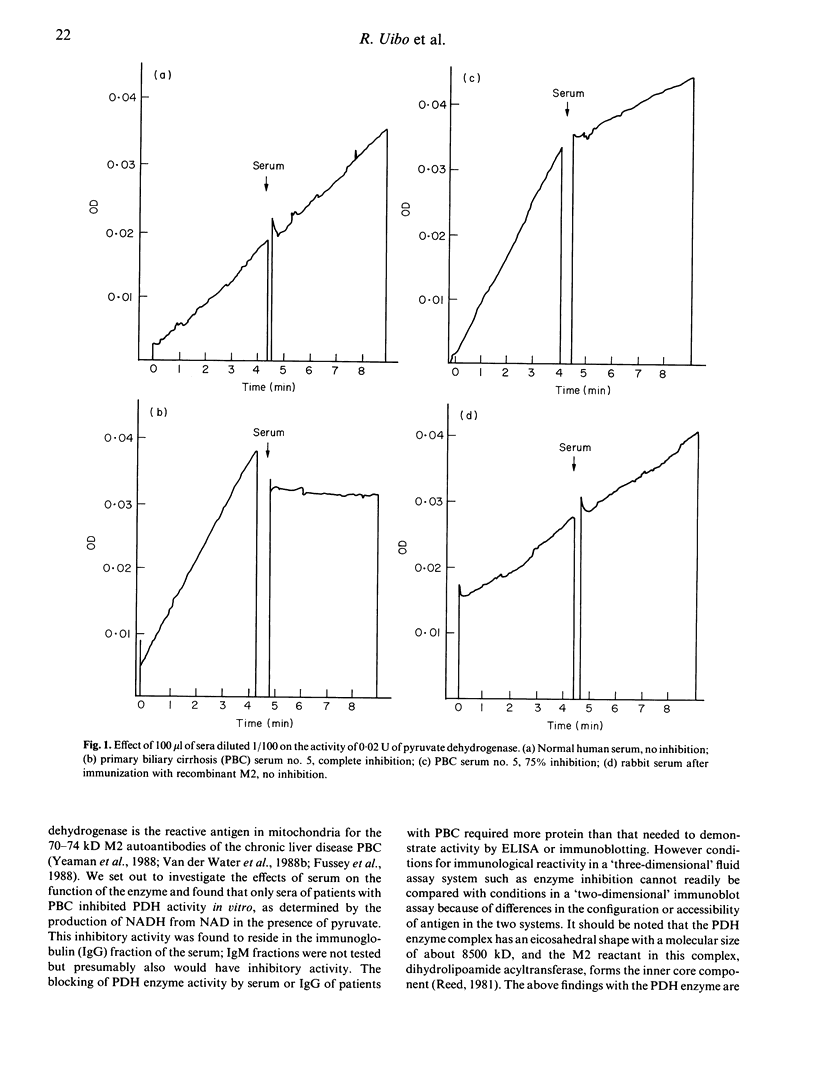
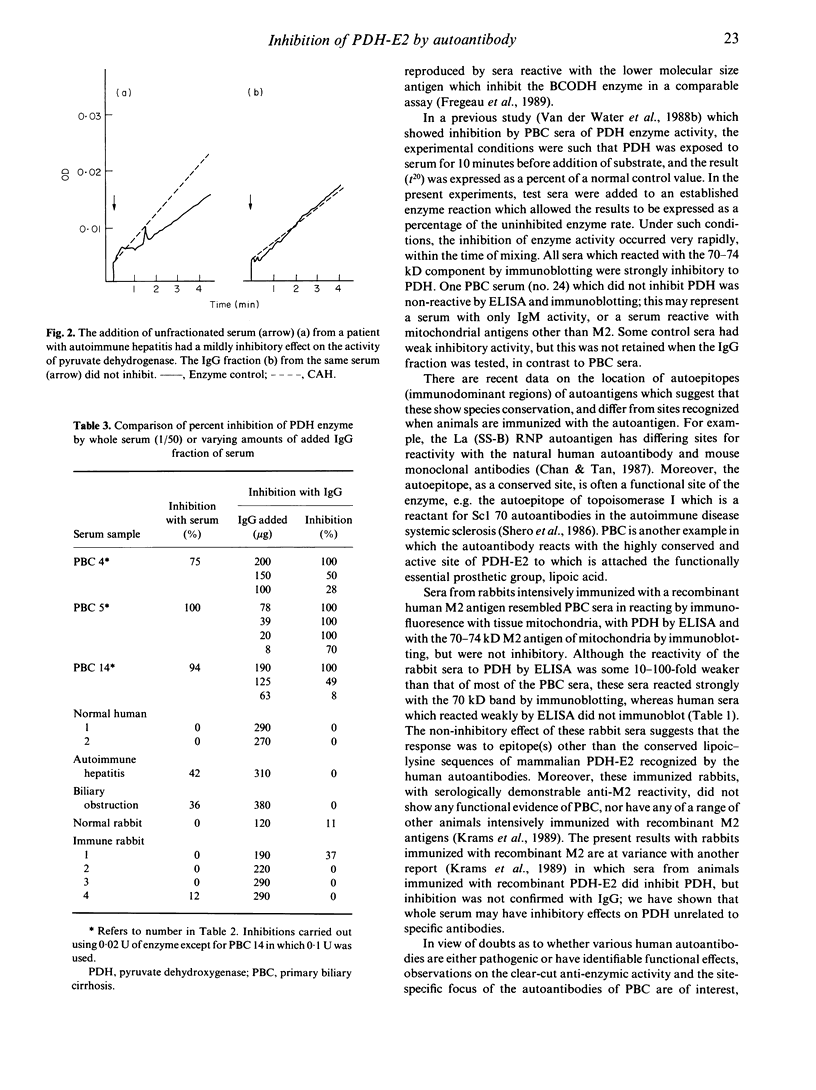
Antibodies to the mitochondrial autoantigen M2, characteristic of the autoimmune liver disease primary biliary cirrhosis (PBC), react with the E2 subunit of the pyruvate dehydrogenase enzyme (PDH-E2). We examined the effect of disease sera on the enzyme activation catalysed by the PDH complex. Inhibition of enzyme activity was observed in 19 of 24 sera of patients with PBC with a level of greater than 90% inhibition in 14 at a serum dilution of 1/50. The onset of inhibition by serum was rapid, within the time of mixing, and the inhibitory activity was shown to reside in the immunoglobulin fraction of the serum. The immunoglobulin fraction of control sera from patients with other liver diseases (n = 26) and healthy persons (n = 8) failed to produce inhibitory activity. In addition sera from four rabbits, intensively immunized with a recombinant human M2 autoantigen, gave anti-M2 reactions by fluorescence, ELISA and immunoblotting, but did not inhibit the activity of PDH. The failure of experimentally induced M2 antibodies in rabbits to inhibit is interesting in view of the reactivity of the natural M2 autoantibodies of PBC with the highly conserved site on the enzyme which carries the essential lipoic acid cofactor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan E. K., Tan E. M. Human autoantibody-reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987 Dec 1;166(6):1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., McNeilage L. J., Surh C. D., Van de Water J., Spithill T. W., Whittingham S., Gershwin M. E. Primary structure of the human M2 mitochondrial autoantigen of primary biliary cirrhosis: dihydrolipoamide acetyltransferase. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7317–7321. doi: 10.1073/pnas.85.19.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer I. H., Mackay I. R., Jordan T. W., Whittingham S., Marzuki S. Reactivity of anti-mitochondrial autoantibodies in primary biliary cirrhosis: definition of two novel mitochondrial polypeptide autoantigens. J Immunol. 1985 Sep;135(3):1739–1745. [PubMed] [Google Scholar]
- Fregeau D. R., Davis P. A., Danner D. J., Ansari A., Coppel R. L., Dickson E. R., Gershwin M. E. Antimitochondrial antibodies of primary biliary cirrhosis recognize dihydrolipoamide acyltransferase and inhibit enzyme function of the branched chain alpha-ketoacid dehydrogenase complex. J Immunol. 1989 Jun 1;142(11):3815–3820. [PubMed] [Google Scholar]
- Fussey S. P., Bassendine M. F., Fittes D., Turner I. B., James O. F., Yeaman S. J. The E1 alpha and beta subunits of the pyruvate dehydrogenase complex are M2'd' and M2'e' autoantigens in primary biliary cirrhosis. Clin Sci (Lond) 1989 Oct;77(4):365–368. doi: 10.1042/cs0770365. [DOI] [PubMed] [Google Scholar]
- Fussey S. P., Guest J. R., James O. F., Bassendine M. F., Yeaman S. J. Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8654–8658. doi: 10.1073/pnas.85.22.8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Coppel R. L., Mackay I. R. Primary biliary cirrhosis and mitochondrial autoantigens--insights from molecular biology. Hepatology. 1988 Jan-Feb;8(1):147–151. doi: 10.1002/hep.1840080128. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Mackay I. R., Sturgess A., Coppel R. L. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987 May 15;138(10):3525–3531. [PubMed] [Google Scholar]
- Krams S. M., Surh C. D., Coppel R. L., Ansari A., Ruebner B., Gershwin M. E. Immunization of experimental animals with dihydrolipoamide acetyltransferase, as a purified recombinant polypeptide, generates mitochondrial antibodies but not primary biliary cirrhosis. Hepatology. 1989 Mar;9(3):411–416. doi: 10.1002/hep.1840090311. [DOI] [PubMed] [Google Scholar]
- Lindenborn-Fotinos J., Baum H., Berg P. A. Mitochondrial antibodies in primary biliary cirrhosis: species and nonspecies specific determinants of M2 antigen. Hepatology. 1985 Sep-Oct;5(5):763–769. doi: 10.1002/hep.1840050510. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Reed L. J. Pyruvate dehydrogenase complex from bovine kidney and heart. Methods Enzymol. 1982;89(Pt 500):376–386. doi: 10.1016/s0076-6879(82)89067-8. [DOI] [PubMed] [Google Scholar]
- Shero J. H., Bordwell B., Rothfield N. F., Earnshaw W. C. High titers of autoantibodies to topoisomerase I (Scl-70) in sera from scleroderma patients. Science. 1986 Feb 14;231(4739):737–740. doi: 10.1126/science.3003910. [DOI] [PubMed] [Google Scholar]
- Surh C. D., Cooper A. E., Coppel R. L., Leung P., Ahmed A., Dickson R., Gershwin M. E. The predominance of IgG3 and IgM isotype antimitochondrial autoantibodies against recombinant fused mitochondrial polypeptide in patients with primary biliary cirrhosis. Hepatology. 1988 Mar-Apr;8(2):290–295. doi: 10.1002/hep.1840080217. [DOI] [PubMed] [Google Scholar]
- Surh C. D., Danner D. J., Ahmed A., Coppel R. L., Mackay I. R., Dickson E. R., Gershwin M. E. Reactivity of primary biliary cirrhosis sera with a human fetal liver cDNA clone of branched-chain alpha-keto acid dehydrogenase dihydrolipoamide acyltransferase, the 52 kD mitochondrial autoantigen. Hepatology. 1989 Jan;9(1):63–68. doi: 10.1002/hep.1840090110. [DOI] [PubMed] [Google Scholar]
- Van de Water J., Fregeau D., Davis P., Ansari A., Danner D., Leung P., Coppel R., Gershwin M. E. Autoantibodies of primary biliary cirrhosis recognize dihydrolipoamide acetyltransferase and inhibit enzyme function. J Immunol. 1988 Oct 1;141(7):2321–2324. [PubMed] [Google Scholar]
- Van de Water J., Gershwin M. E., Leung P., Ansari A., Coppel R. L. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988 Jun 1;167(6):1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER J. G., DONIACH D., ROITT I. M., SHERLOCK S. SEROLOGICAL TESTS IN DIAGNOSIS OF PRIMARY BILIARY CIRRHOSIS. Lancet. 1965 Apr 17;1(7390):827–831. doi: 10.1016/s0140-6736(65)91372-3. [DOI] [PubMed] [Google Scholar]
- Whittingam S., Mackay I. R. Laboratory methods for diagnosis of autoimmune disease. Med J Aust. 1969 Jun 7;1(23):1200–1205. doi: 10.5694/j.1326-5377.1969.tb62269.x. [DOI] [PubMed] [Google Scholar]
- Yeaman S. J., Fussey S. P., Danner D. J., James O. F., Mutimer D. J., Bassendine M. F. Primary biliary cirrhosis: identification of two major M2 mitochondrial autoantigens. Lancet. 1988 May 14;1(8594):1067–1070. doi: 10.1016/s0140-6736(88)91894-6. [DOI] [PubMed] [Google Scholar]


