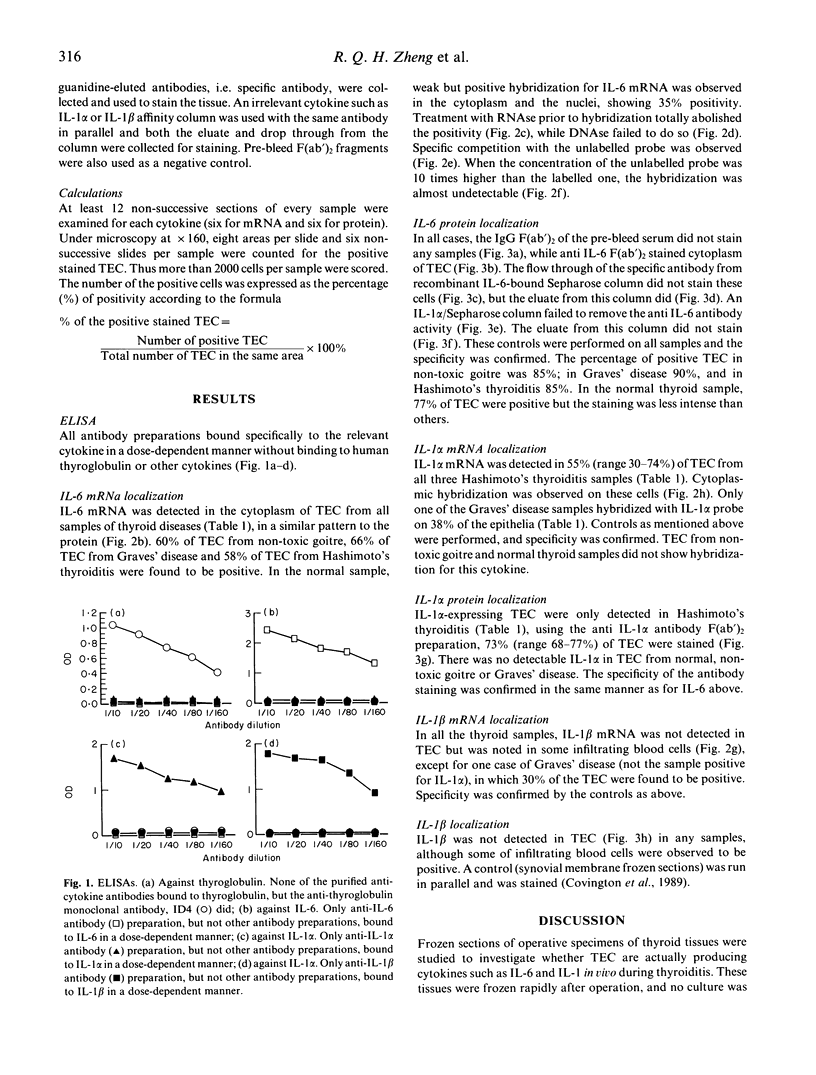
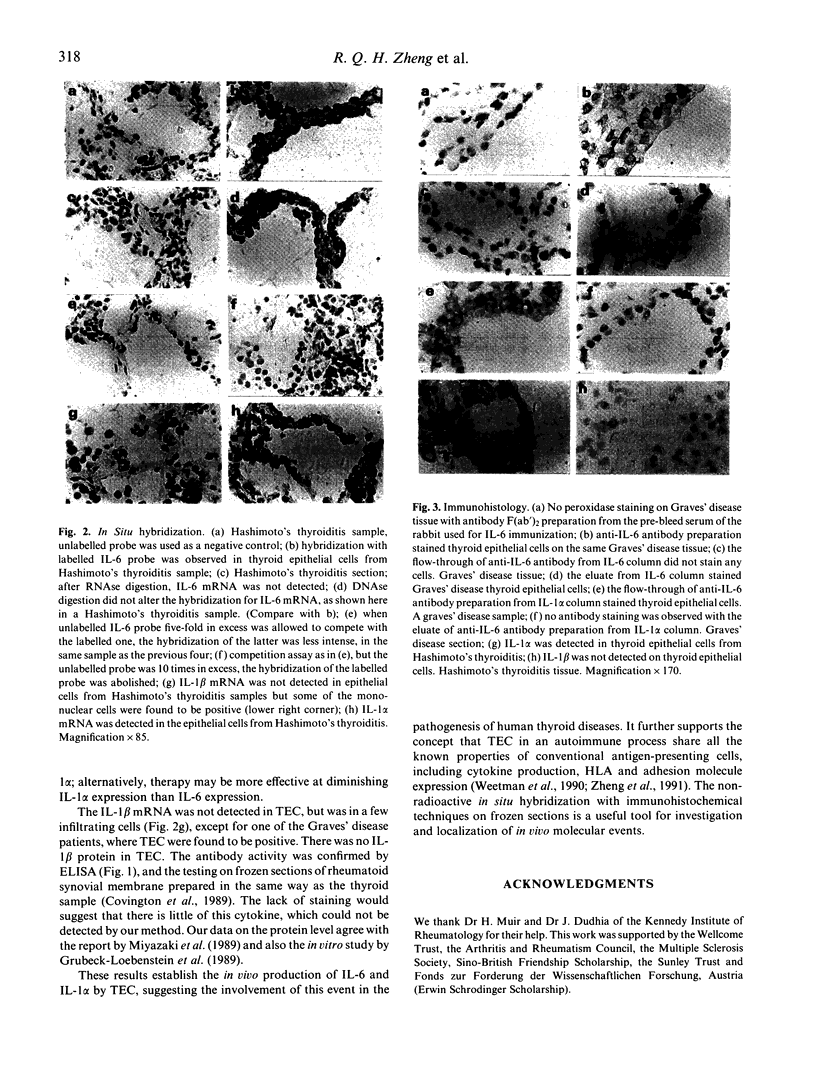
Abstract
Human endocrine thyroid epithelial cells have been described to produce cytokines in vitro. In order to determine whether they do so in vivo during thyroiditis, parallel studies on mRNA expression with a non-radioactive in situ hybridization technique and immunohistochemical detection for the protein were performed on frozen sections of thyroid samples from autoimmune thyroiditis (Graves' disease and Hashimoto's thyroiditis), non-toxic goitre and normal thyroid tissue. cDNA probes were sulphonated and their hybridization with mRNA was detected with a sulphonyl-specific monoclonal antibody. This signal was amplified and visualized with the alkaline phosphatase-anti-alkaline phosphatase (APAAP) system. The protein products were detected with immuno-purified rabbit F(ab')2 antibody fragments recognizing recombinant human cytokines, visualized by the immunoperoxidase technique. Each sample was studied at the two levels. Both interleukin-6 mRNA and protein were found in the endocrine cells. There was no obvious difference between autoimmune thyroiditis and non-toxic goitre. However, normal thyroid epithelial cells produced less interleukin-6. Interleukin-1 alpha mRNA and its protein were found in epithelial cells from Hashimoto's thyroiditis samples, but not in the others, except one Graves' disease sample, in which only mRNA was detected. Interleukin-1 beta was not detected in these cells, its mRNA was only found in one of the Graves' disease samples. These cytokines were also detected in some infiltrating cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroja M. L., Ceuppens J. L., Van Damme J., Billiau A. Cooperation between an anti-T cell (anti-CD28) monoclonal antibody and monocyte-produced IL-6 in the induction of T cell responsiveness to IL-2. J Immunol. 1988 Sep 1;141(5):1502–1507. [PubMed] [Google Scholar]
- Budowsky E. I., Sverdlov E. D., Monastyrskaya G. S. New method of selective and rapid modification of the cytidine residues. FEBS Lett. 1972 Sep 1;25(1):201–204. doi: 10.1016/0014-5793(72)80485-x. [DOI] [PubMed] [Google Scholar]
- Chan C. T., Byfield P. G., Himsworth R. L., Shepherd P. Human autoantibodies to thyroglobulin are directed towards a restricted number of human specific epitopes. Clin Exp Immunol. 1987 Sep;69(3):516–523. [PMC free article] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Farquharson M., Harvie R., McNicol A. M. Detection of messenger RNA using a digoxigenin end labelled oligodeoxynucleotide probe. J Clin Pathol. 1990 May;43(5):424–428. doi: 10.1136/jcp.43.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Herzbeck H., Schlüter C., Flad H. D. Immunoenzymatic assessment of IL-1 beta mRNA by in situ hybridization using sulphonated probes. Lymphokine Res. 1989 Fall;8(3):239–243. [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Buchan G., Chantry D., Kassal H., Londei M., Pirich K., Barrett K., Turner M., Waldhausl W., Feldmann M. Analysis of intrathyroidal cytokine production in thyroid autoimmune disease: thyroid follicular cells produce interleukin-1 alpha and interleukin-6. Clin Exp Immunol. 1989 Sep;77(3):324–330. [PMC free article] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Londei M., Greenall C., Pirich K., Kassal H., Waldhäusl W., Feldmann M. Pathogenetic relevance of HLA class II expressing thyroid follicular cells in nontoxic Goiter and in Graves' disease. J Clin Invest. 1988 May;81(5):1608–1614. doi: 10.1172/JCI113495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Stern A. S., Hellmann C. P., Vitek M. P., DeChiara T. M., Benjamin W. R., Collier K. J., Dukovich M., Familletti P. C. Recombinant human interleukin 1 alpha: purification and biological characterization. J Immunol. 1986 Apr 1;136(7):2492–2497. [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Hirose W., Kawagoe M., Hara M., Kitani A., Hirose T., Norioka K., Harigai M., Nakamura H. Production of thymocyte-stimulating activity by cultured human thyroid epithelial cells. Clin Exp Immunol. 1987 Oct;70(1):102–109. [PMC free article] [PubMed] [Google Scholar]
- Lebacq P., Squalli D., Duchenne M., Pouletty P., Joannes M. A new sensitive non-isotopic method using sulfonated probes to detect picogram quantities of specific DNA sequences on blot hybridization. J Biochem Biophys Methods. 1988 Mar;15(5):255–266. doi: 10.1016/0165-022x(88)90013-9. [DOI] [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Miyazaki A., Hanafusa T., Itoh N., Miyagawa J., Kono N., Tarui S., Kiyotaki C., Yoshizaki K. Demonstration of interleukin-1 beta on perifollicular endothelial cells in the thyroid glands of patients with Graves' disease. J Clin Endocrinol Metab. 1989 Oct;69(4):738–744. doi: 10.1210/jcem-69-4-738. [DOI] [PubMed] [Google Scholar]
- Poverenny A. M., Podgorodnichenko V. K., Bryksina L. E., Monastyrskaya G. S., Sverdlov E. D. Immunochemical approaches to DNA structure investigation--I. Immunochemical identification of the product of cytosine modification with bisulphite and O-methylhydroxylamine mixture. Mol Immunol. 1979 May;16(5):313–316. doi: 10.1016/0161-5890(79)90132-9. [DOI] [PubMed] [Google Scholar]
- Pringle J. H., Primrose L., Kind C. N., Talbot I. C., Lauder I. In situ hybridization demonstration of poly-adenylated RNA sequences in formalin-fixed paraffin sections using a biotinylated oligonucleotide poly d(T) probe. J Pathol. 1989 Aug;158(4):279–286. doi: 10.1002/path.1711580403. [DOI] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Verdlov E. D., Monastyrskaya G. S., Guskova L. I., Levitan T. L., Sheichenko V. I., Budowsky E. I. Modification of cytidine residues with a bisulfite-O-methylhydroxylamine mixture. Biochim Biophys Acta. 1974 Mar 8;340(2):153–165. [PubMed] [Google Scholar]
- Weetman A. P., Freeman M., Borysiewicz L. K., Makgoba M. W. Functional analysis of intercellular adhesion molecule-1-expressing human thyroid cells. Eur J Immunol. 1990 Feb;20(2):271–275. doi: 10.1002/eji.1830200207. [DOI] [PubMed] [Google Scholar]
- Zeheb R., Chang V., Orr G. A. An analytical method for the selective retrieval of iminobiotin-derivatized plasma membrane proteins. Anal Biochem. 1983 Feb 15;129(1):156–161. doi: 10.1016/0003-2697(83)90063-5. [DOI] [PubMed] [Google Scholar]




