Abstract
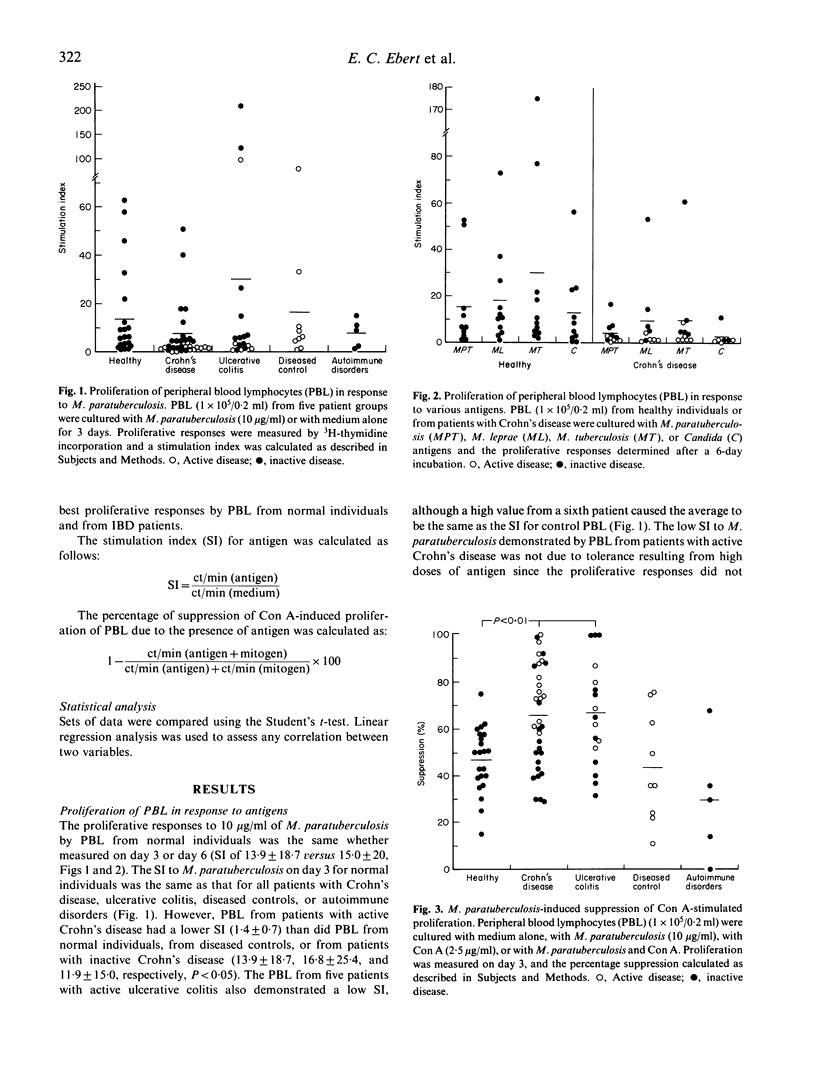
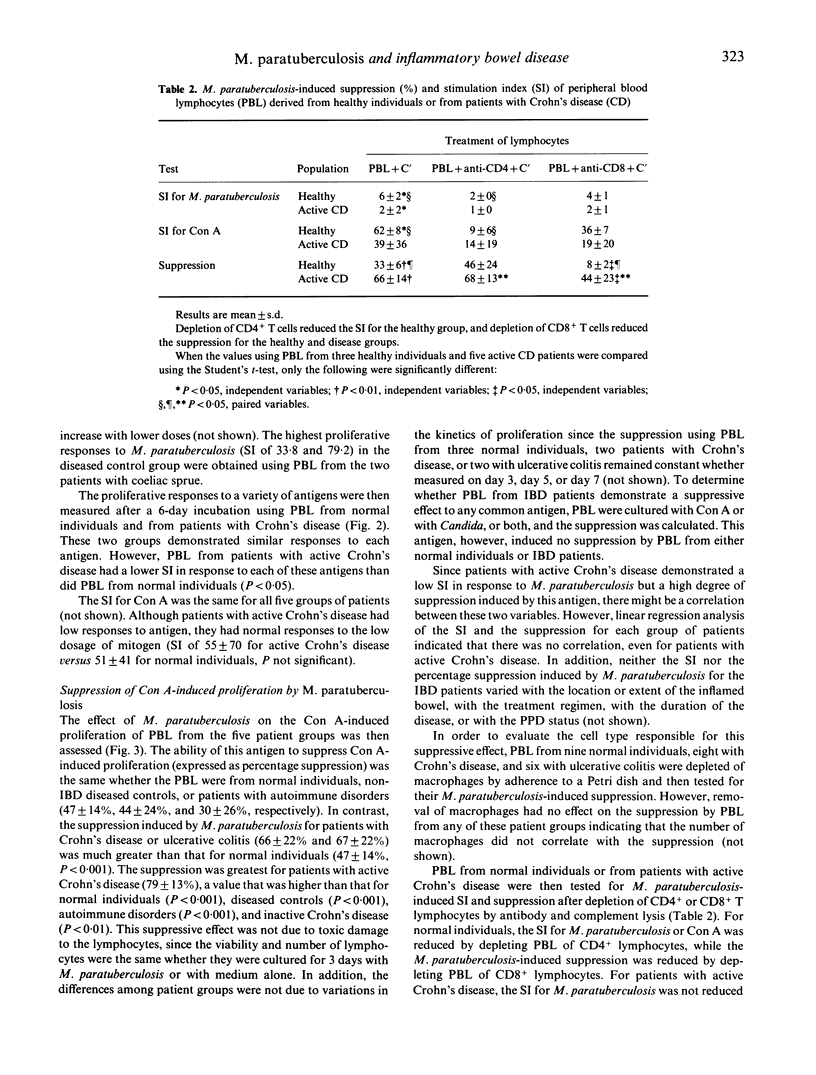
We studied the M. paratuberculosis-induced proliferation and suppressor cell generation by peripheral blood lymphocytes from patients with inflammatory bowel disease. Peripheral blood lymphocytes were separated from 33 patients with Crohn's disease, 18 with ulcerative colitis, nine with other intestinal diseases, and five with autoimmune disorders. Proliferation of peripheral blood lymphocytes from normal individuals in response to 10 micrograms/ml of M. paratuberculosis antigen was reduced by depletion of CD4+ T cells. The ability of M. paratuberculosis antigen to suppress concanavalin A-induced proliferation (expressed as a percentage suppression) was reduced by depletion of CD8+ T cells. This suppression was the same whether peripheral blood lymphocytes were from normal individuals, patients with intestinal diseases other than inflammatory bowel disease, or patients with autoimmune disorders (47 +/- 14%, 44 +/- 24%, and 30 +/- 26%, respectively). In contrast, the suppression induced by M. paratuberculosis for patients with Crohn's disease and ulcerative colitis (66 +/- 22% and 67 +/- 22%) was much greater than that for normal individuals (P less than 0.001). In particular, lymphocytes from patients with active Crohn's disease demonstrated little proliferation in response to this antigen but marked suppressor activity (79 +/- 13%). How the immunomodulatory effects of this antigen relate to the pathogenesis of the inflammatory bowel diseases remains to be determined.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitstead J. G., Ewan P. W. Concanavalin A-induced suppressor cells. J Clin Lab Immunol. 1984 Jan;13(1):1–10. [PubMed] [Google Scholar]
- Bloom B. R. Learning from leprosy: a perspective on immunology and the Third World. J Immunol. 1986 Jul 1;137(1):i–x. [PubMed] [Google Scholar]
- Chao L. P., Steele J., Rodrigues C., Lennard-Jones J., Stanford J. L., Spiliadis C., Rook G. A. Specificity of antibodies secreted by hybridomas generated from activated B cells in the mesenteric lymph nodes of patients with inflammatory bowel disease. Gut. 1988 Jan;29(1):35–40. doi: 10.1136/gut.29.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Thayer W. R., Merkal R. S., Coutu J. A. Possible role of mycobacteria in inflammatory bowel disease. I. An unclassified Mycobacterium species isolated from patients with Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1073–1079. doi: 10.1007/BF01317078. [DOI] [PubMed] [Google Scholar]
- De Dombal F. T., Burton I. L., Clamp S. E., Goligher J. C. Short-term course and prognosis of Crohn's disease. Gut. 1974 Jun;15(6):435–443. doi: 10.1136/gut.15.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert E. C. Proliferative responses of human intraepithelial lymphocytes to various T-cell stimuli. Gastroenterology. 1989 Dec;97(6):1372–1381. doi: 10.1016/0016-5085(89)90379-x. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Markesich D. C., Yoshimura H. H. Mycobacteria and inflammatory bowel disease. Results of culture. Gastroenterology. 1987 Feb;92(2):436–442. doi: 10.1016/0016-5085(87)90139-9. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- McClure H. M., Chiodini R. J., Anderson D. C., Swenson R. B., Thayer W. R., Coutu J. A. Mycobacterium paratuberculosis infection in a colony of stumptail macaques (Macaca arctoides). J Infect Dis. 1987 May;155(5):1011–1019. doi: 10.1093/infdis/155.5.1011. [DOI] [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Schultz M. G., Rieder H. L., Hersh T., Riepe S. Remission of Crohn's disease with antimycobacterial chemotherapy. Lancet. 1987 Dec 12;2(8572):1391–1392. doi: 10.1016/s0140-6736(87)91276-1. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A. Mycobacteria and autoimmunity. Immunol Today. 1988 Jun;9(6):178–182. doi: 10.1016/0167-5699(88)91294-7. [DOI] [PubMed] [Google Scholar]
- Thayer W. R., Jr, Coutu J. A., Chiodini R. J., Van Kruiningen H. J., Merkal R. S. Possible role of mycobacteria in inflammatory bowel disease. II. Mycobacterial antibodies in Crohn's disease. Dig Dis Sci. 1984 Dec;29(12):1080–1085. doi: 10.1007/BF01317079. [DOI] [PubMed] [Google Scholar]
- Warren J. B., Rees H. C., Cox T. M. Remission of Crohn's disease with tuberculosis chemotherapy. N Engl J Med. 1986 Jan 16;314(3):182–182. doi: 10.1056/NEJM198601163140314. [DOI] [PubMed] [Google Scholar]


