Abstract
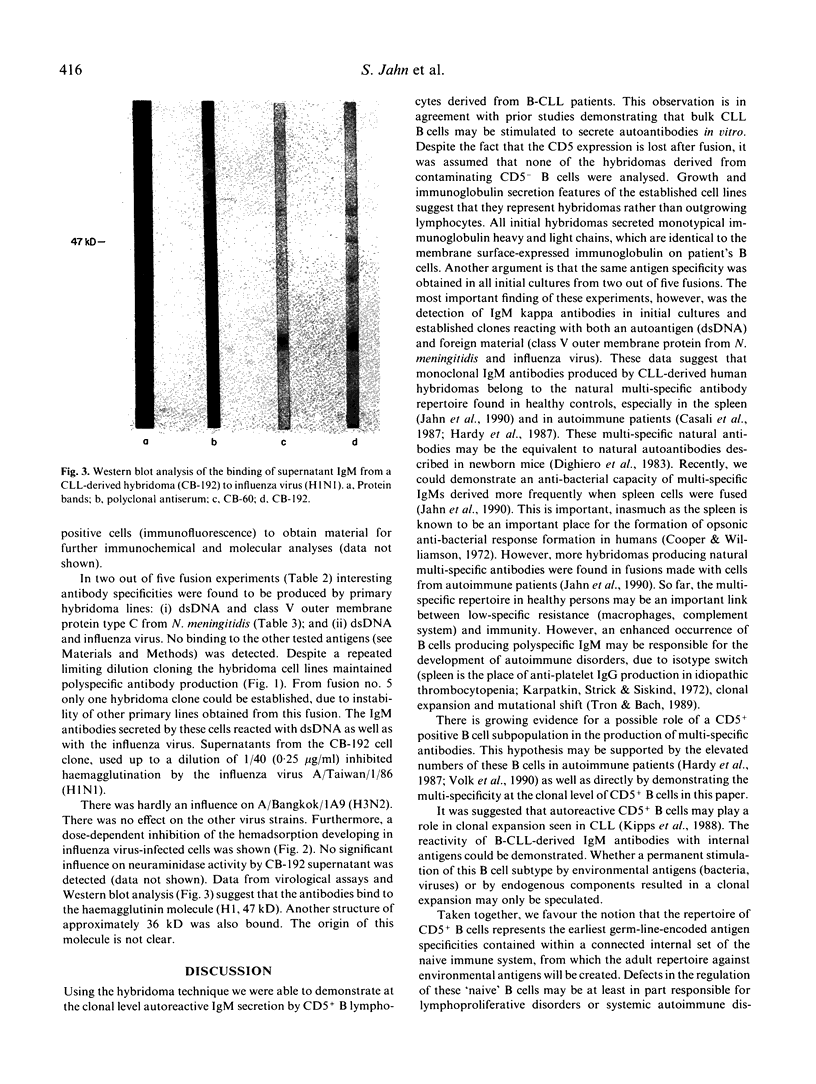
Great numbers of CD5+ B lymphocytes were detected in the peripheral blood of patients with B-CLL. To study the antibody repertoire of this immune cell subpopulation on a monoclonal level, we fused the lymphocytes derived from five different donors to a highly efficient HAT-sensitive heteromyeloma line (CB-F7). A fusion frequency of up to 10-5 allowed us to analyse hundreds of initial hybridoma lines per fusion. In all culture supernatants in three out of five fusions IgM lambda antibodies were detected, in two experiments only IgM kappa was measured, suggesting monoclonality of the primary hybridoma cell lines. The later fusions resulted in hybridomas producing multi-specific antibodies against both an autoantigen and an infectious agent: (i) dsDNA/influenza virus haemagglutinin; (ii) dsDNA/class V outer membrane protein type C from Neisseria meningitidis. However, no antibodies of the described specificity were detected in blood sera of patients, indicating a `switch-on' of the immunoglobulin secretion capacity of malignant B cells during fusion to a myeloma partner. We discuss the results as further evidence for the natural multi-reactive antibody repertoire of CD5+ B cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A revised system of nomenclature for influenza viruses. Bull World Health Organ. 1971;45(1):119–124. [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Neibert M., Crowe B. A., Strittmatter W., Kusecek B., Weyse E., Walsh M. J., Slawig B., Morelli G., Moll A. Purification and characterization of eight class 5 outer membrane protein variants from a clone of Neisseria meningitidis serogroup A. J Exp Med. 1988 Aug 1;168(2):507–525. doi: 10.1084/jem.168.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. J., Williamson R. C. Splenectomy: indications, hazards and alternatives. Br J Surg. 1984 Mar;71(3):173–180. doi: 10.1002/bjs.1800710302. [DOI] [PubMed] [Google Scholar]
- Dersimonian H., Schwartz R. S., Barrett K. J., Stollar B. D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987 Oct 1;139(7):2496–2501. [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Mazié J. C., Rouyre S., Butler-Browne G. S., Whalen R. G., Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983 Nov;131(5):2267–2272. [PubMed] [Google Scholar]
- Foon K. A., Todd R. F., 3rd Immunologic classification of leukemia and lymphoma. Blood. 1986 Jul;68(1):1–31. [PubMed] [Google Scholar]
- Grunow R., Jahn S., Porstmann T., Kiessig S. S., Steinkellner H., Steindl F., Mattanovich D., Gürtler L., Deinhardt F., Katinger H. The high efficiency, human B cell immortalizing heteromyeloma CB-F7. Production of human monoclonal antibodies to human immunodeficiency virus. J Immunol Methods. 1988 Feb 10;106(2):257–265. doi: 10.1016/0022-1759(88)90206-2. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Shimizu M., Yamasaki K., Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987 Apr 3;236(4797):81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Jones N. H., Strominger J. L., Herzenberg L. A. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: molecular homology to its human counterpart Leu-1/T1 (CD5). Proc Natl Acad Sci U S A. 1987 Jan;84(1):204–208. doi: 10.1073/pnas.84.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn S., Grunow R., Kiessig S. T., Settmacher U., Von Baehr R. Strategies in the development of human monoclonal antibodies. Dev Biol Stand. 1990;71:3–7. [PubMed] [Google Scholar]
- Jahn S., Kiessig S. T., Lukowsky A., Volk H. D., Porstmann T., Grunow R., von Baehr R. Pokeweed mitogen induced synthesis of human IgG and IgM in vitro. Technical aspects and the role of adherent cells. Biomed Biochim Acta. 1986;45(4):467–476. [PubMed] [Google Scholar]
- Karpatkin S., Strick N., Siskind G. W. Detection of splenic anti-platelet antibody synthesis in idiopathic autoimmune thrombocytopenic purpura (ATP). Br J Haematol. 1972 Aug;23(2):167–176. doi: 10.1111/j.1365-2141.1972.tb03470.x. [DOI] [PubMed] [Google Scholar]
- Kiessig S. T., Jahn S., Porstmann T., von Baehr R. In vitro Immunisierung zur Erzeugung von Antikörpern gegen Tetanustoxin bzw. -toxoid. 1. Nachweissysteme für in vitro synthetisierte spezifische Immunglobuline. Strategien beim Testaufbau. Allerg Immunol (Leipz) 1987;33(2):79–87. [PubMed] [Google Scholar]
- Kipps T. J., Tomhave E., Chen P. P., Carson D. A. Autoantibody-associated kappa light chain variable region gene expressed in chronic lymphocytic leukemia with little or no somatic mutation. Implications for etiology and immunotherapy. J Exp Med. 1988 Mar 1;167(3):840–852. doi: 10.1084/jem.167.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehl M., Starke R., Kissig B., Grunow R., Jahn S., Schmidt G., von Baehr R. Chemische Charakterisierung des Escherichia coli K 1 Kapselpolysaccharides. J Basic Microbiol. 1989;29(4):221–232. doi: 10.1002/jobm.3620290408. [DOI] [PubMed] [Google Scholar]
- Prösch S., Heider H., Schroeder C., Krüger D. H. Mutations in the hemagglutinin gene associated with influenza virus resistance to norakin. Arch Virol. 1988;102(1-2):125–129. doi: 10.1007/BF01315569. [DOI] [PubMed] [Google Scholar]
- Prösch S., Heider H., Schroeder C., Krüger D. H. Mutations in the hemagglutinin gene associated with influenza virus resistance to norakin. Arch Virol. 1988;102(1-2):125–129. doi: 10.1007/BF01315569. [DOI] [PubMed] [Google Scholar]
- Sanz I., Capra J. D. The genetic origin of human autoantibodies. J Immunol. 1988 May 15;140(10):3283–3285. [PubMed] [Google Scholar]
- Sanz I., Casali P., Thomas J. W., Notkins A. L., Capra J. D. Nucleotide sequences of eight human natural autoantibody VH regions reveals apparent restricted use of VH families. J Immunol. 1989 Jun 1;142(11):4054–4061. [PubMed] [Google Scholar]
- Tron F., Bach J. F. Molecular and genetic characteristics of pathogenic autoantibodies. J Autoimmun. 1989 Aug;2(4):311–320. doi: 10.1016/0896-8411(89)90158-3. [DOI] [PubMed] [Google Scholar]



