Abstract
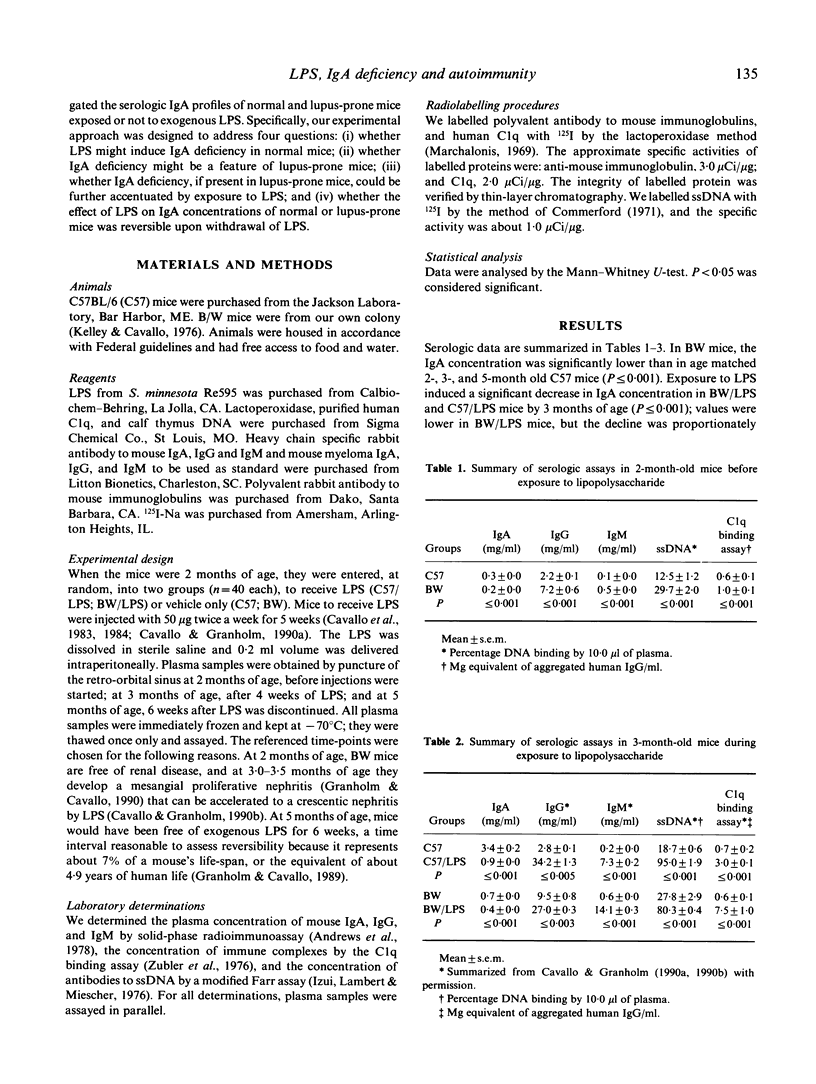
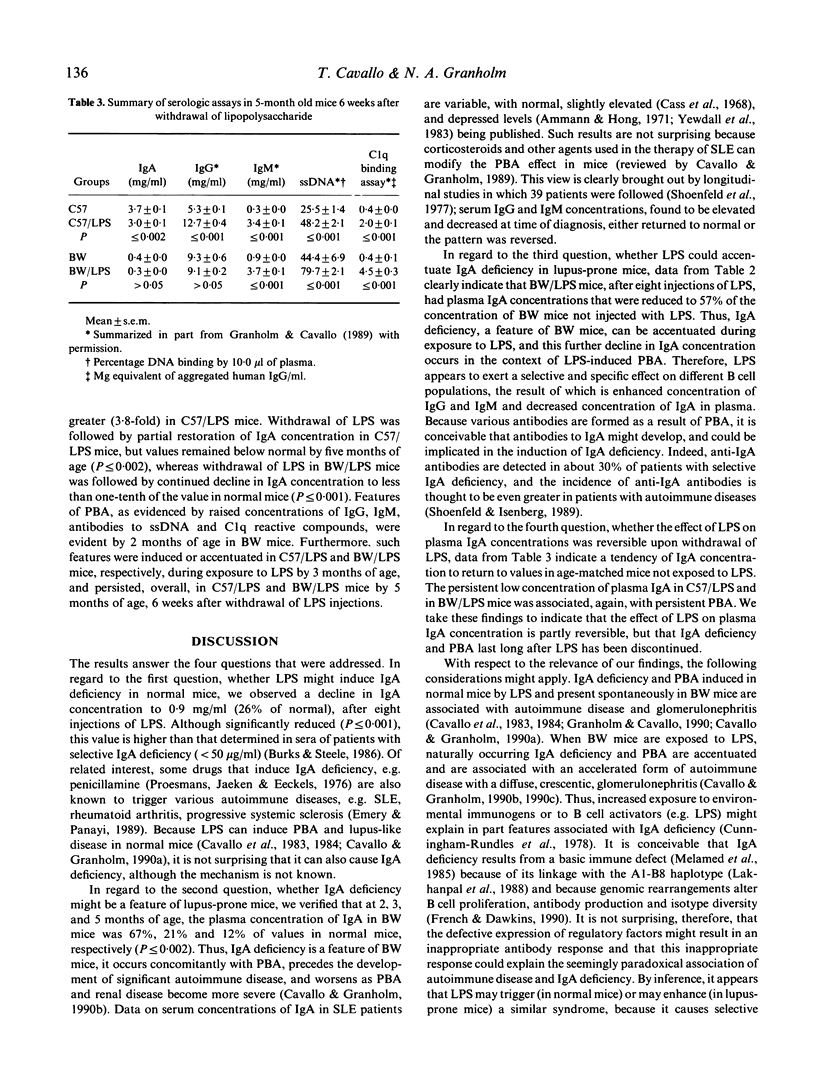
Polyclonal B cell activation (PBA) and autoimmune disease can be induced in immunologically normal mice, or enhanced in lupus-prone mice, by bacterial lipopolysaccharide (LPS). Because immune defects are common in autoimmune diseases and IgA deficiency is prevalent in patients with systemic lupus erythematosus, we investigated: (i) whether LPS might induce IgA deficiency in normal mice; (ii) whether IgA deficiency might be a feature in lupus-prone mice; (iii) whether, if present in lupus-prone mice, IgA deficiency could be further accentuated by LPS; and (iv) whether the effects of LPS on IgA concentrations of normal and lupus-prone mice might be reversible upon withdrawal of LPS. We injected normal (C57BL/6) and lupus-prone (NZB/W) mice with 50 micrograms of LPS from Salmonella minnesota Re595 twice a week for 5 weeks and then discontinued LPS for 6 weeks. We determined the concentrations of plasma immunoglobulins, DNA antibodies, and circulating immune complexes before, during, and after mice were exposed to LPS. Our results indicate that: (i) LPS induces IgA deficiency in normal mice concurrently with PBA; (ii) IgA deficiency is a feature of lupus-prone mice; (iii) LPS accentuates naturally occurring PBA and IgA deficiency in lupus-prone mice; and (iv) LPS induced, or LPS enhanced, IgA deficiency and PBA in normal and lupus-prone mice persist long after withdrawal of LPS. Thus, LPS triggers or enhances autoimmune disease by a mechanism that involves in part PBA with selective increase (IgG, IgM) and concurrent decrease (IgA) of specific isotypes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann A. J., Hong R. Selective IgA deficiency: presentation of 30 cases and a review of the literature. Medicine (Baltimore) 1971 May;50(3):223–236. [PubMed] [Google Scholar]
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass R. M., Mongan E. S., Jacox R. F., Vaughan J. H. Immunoglobulins G, A, and M in systemic lupus erythematosus. Relationship to serum complement titer, latex titer, antinuclear antibody, and manifestations of clinical disease. Ann Intern Med. 1968 Oct;69(4):749–756. doi: 10.7326/0003-4819-69-4-749. [DOI] [PubMed] [Google Scholar]
- Cavallo T., Goldman M., Graves K., Lambert P. H. Altered glomerular permeability in the early phase of immune complex nephritis. Kidney Int. 1983 Nov;24(5):632–637. doi: 10.1038/ki.1983.204. [DOI] [PubMed] [Google Scholar]
- Cavallo T., Goldman M., Lambert P. H. Animal model of human disease. Proliferative glomerulonephritis associated with polyclonal B-cell activation. Am J Pathol. 1984 Feb;114(2):346–348. [PMC free article] [PubMed] [Google Scholar]
- Cavallo T., Granholm N. A. Accelerated (proliferative) lupus nephritis. Am J Pathol. 1990 Dec;137(6):1549–1551. [PMC free article] [PubMed] [Google Scholar]
- Cavallo T., Granholm N. A. Bacterial lipopolysaccharide transforms mesangial into proliferative lupus nephritis without interfering with processing of pathogenic immune complexes in NZB/W mice. Am J Pathol. 1990 Oct;137(4):971–978. [PMC free article] [PubMed] [Google Scholar]
- Cavallo T., Granholm N. A. Repeated exposure to bacterial lipopolysaccharide interferes with disposal of pathogenic immune complexes in mice. Clin Exp Immunol. 1990 Feb;79(2):253–259. doi: 10.1111/j.1365-2249.1990.tb05187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Gronowicz E. Genetical control of B-cell responses. III. Requirement for functional mitogenicity of the antigen in thymus-independent specific responses. J Exp Med. 1975 Apr 1;141(4):753–760. [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975;21:113–236. doi: 10.1016/s0065-2776(08)60220-5. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles C., Brandeis W. E., Good R. A., Day N. K. Milk precipitins, circulating immune complexes, and IgA deficiency. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3387–3389. doi: 10.1073/pnas.75.7.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie G. J., Minh M. G., Mignon-Conte M. A., Hass S., Lambert P. H., Conte J. J. Acceleration of glomerulonephritis in NZB x NZW mice by early immunization with DNA and injection of bacterial lipopolysaccharide. Experimental approach to the treatment of lupus nephritis by use of the accelerated model of NZB x NZW mouse disease. J Clin Lab Immunol. 1980 Sep;4(2):103–106. [PubMed] [Google Scholar]
- French M. A., Dawkins R. L. Central MHC genes, IgA deficiency and autoimmune disease. Immunol Today. 1990 Aug;11(8):271–274. doi: 10.1016/0167-5699(90)90110-u. [DOI] [PubMed] [Google Scholar]
- Granholm N. A., Cavallo T. Defective disposal of immune complexes and polyclonal B cell activation persist long after exposure to bacterial lipopolysaccharide in mice. Lab Invest. 1989 Nov;61(5):504–508. [PubMed] [Google Scholar]
- Hang L., Slack J. H., Amundson C., Izui S., Theofilopoulos A. N., Dixon F. J. Induction of murine autoimmune disease by chronic polyclonal B cell activation. J Exp Med. 1983 Mar 1;157(3):874–883. doi: 10.1084/jem.157.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Fournié G. J., Türler H., Miescher P. A. Features of systemic lupus erythematosus in mice injected with bacterial lipopolysaccharides: identificantion of circulating DNA and renal localization of DNA-anti-DNA complexes. J Exp Med. 1977 May 1;145(5):1115–1130. doi: 10.1084/jem.145.5.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Miescher P. A. Determination of anti-DNA antibodies by a modified 125I-labelled DNA-binding test. Elimination of non-specific binding of DNA to non-immunoglobulin basic proteins by using an anionic detergent. Clin Exp Immunol. 1976 Dec;26(3):425–430. [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Lawton A. R. B lymphocyte differentiation induced by lipopolysaccharide. I. Generation of cells synthesizing four major immunoglobulin classes. J Immunol. 1975 Sep;115(3):671–676. [PubMed] [Google Scholar]
- Kelley V. E., Cavallo T. An ultrastructural study of the glomerular slit diaphragm in New Zealand black/white mice. Lab Invest. 1976 Sep;35(3):213–220. [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhanpal S., O'Duffy J. D., Homburger H. A., Moore S. B. Evidence for linkage of IgA deficiency with the major histocompatibility complex. Mayo Clin Proc. 1988 May;63(5):461–465. doi: 10.1016/s0025-6196(12)65643-2. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed I., Zakuth V., Kark J. O., Spirer Z. The immune system in isolated IgA deficiency. J Clin Lab Immunol. 1985 Aug;17(4):163–166. [PubMed] [Google Scholar]
- Pobor G., Pettersson S., Bandeira A., Martinez-A C., Coutinho A. B lymphocyte activation upon exclusive recognition of major histocompatibility antigens by T helper cells. Eur J Immunol. 1984 Mar;14(3):222–227. doi: 10.1002/eji.1830140305. [DOI] [PubMed] [Google Scholar]
- Proesmans W., Jaeken J., Eeckels R. D-penicillamine-induced IgA deficiency in Wilson's disease. Lancet. 1976 Oct 9;2(7989):804–805. doi: 10.1016/s0140-6736(76)90641-3. [DOI] [PubMed] [Google Scholar]
- Scheid M. P., Goldstein G., Hammerling U., Boyse E. A. Lymphocyte differentiation from precursor cells in vitro. Ann N Y Acad Sci. 1975 Feb 28;249:531–540. doi: 10.1111/j.1749-6632.1975.tb29102.x. [DOI] [PubMed] [Google Scholar]
- Severinson E., Bergstedt-Lindqvist S., van der Loo W., Fernandez C. Characterization of the IgG response induced by polyclonal B cell activators. Immunol Rev. 1982;67:73–85. doi: 10.1111/j.1600-065x.1982.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Pick A. I., Danziger Y., Kalaczi I., Frolichman R., Pinkhas J. Immunoglobulin changes in SLE. Ann Allergy. 1977 Aug;39(2):99–101. [PubMed] [Google Scholar]
- Sieckmann D. G. The use of anti-immunoglobulins to induce a signal for cell division in B lymphocytes via their membrane IgM and IgD. Immunol Rev. 1980;52:181–210. doi: 10.1111/j.1600-065x.1980.tb00335.x. [DOI] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Yewdall V., Cameron J. S., Nathan A. W., Neild G., Ogg C. S., Williams D. G. Systemic lupus erythematosus and IgA deficiency. J Clin Lab Immunol. 1983 Jan;10(1):13–18. [PubMed] [Google Scholar]
- Zubler R. H., Lange G., Lambert P. H., Miescher P. A. Detection of immune complexes in unheated sera by modified 125I-Clq binding test. Effect of heating on the binding of Clq by immune complexes and application of the test to systemic lupus erythematosus. J Immunol. 1976 Jan;116(1):232–235. [PubMed] [Google Scholar]


