Abstract
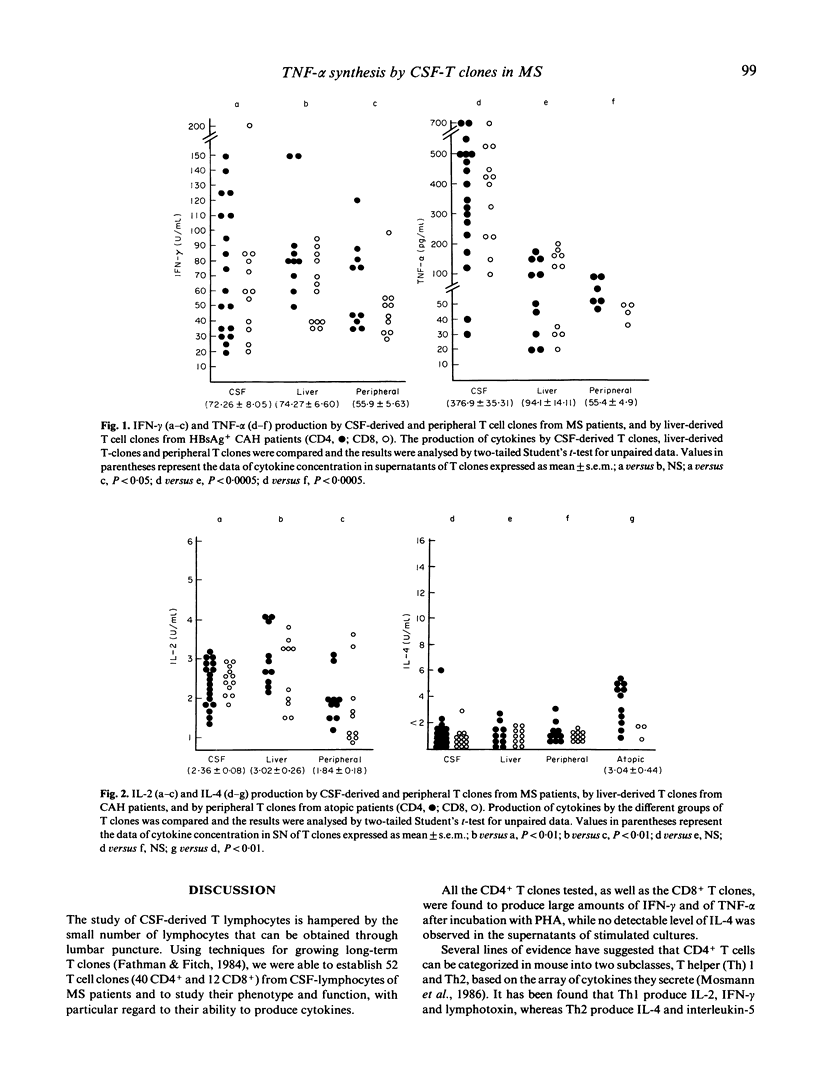
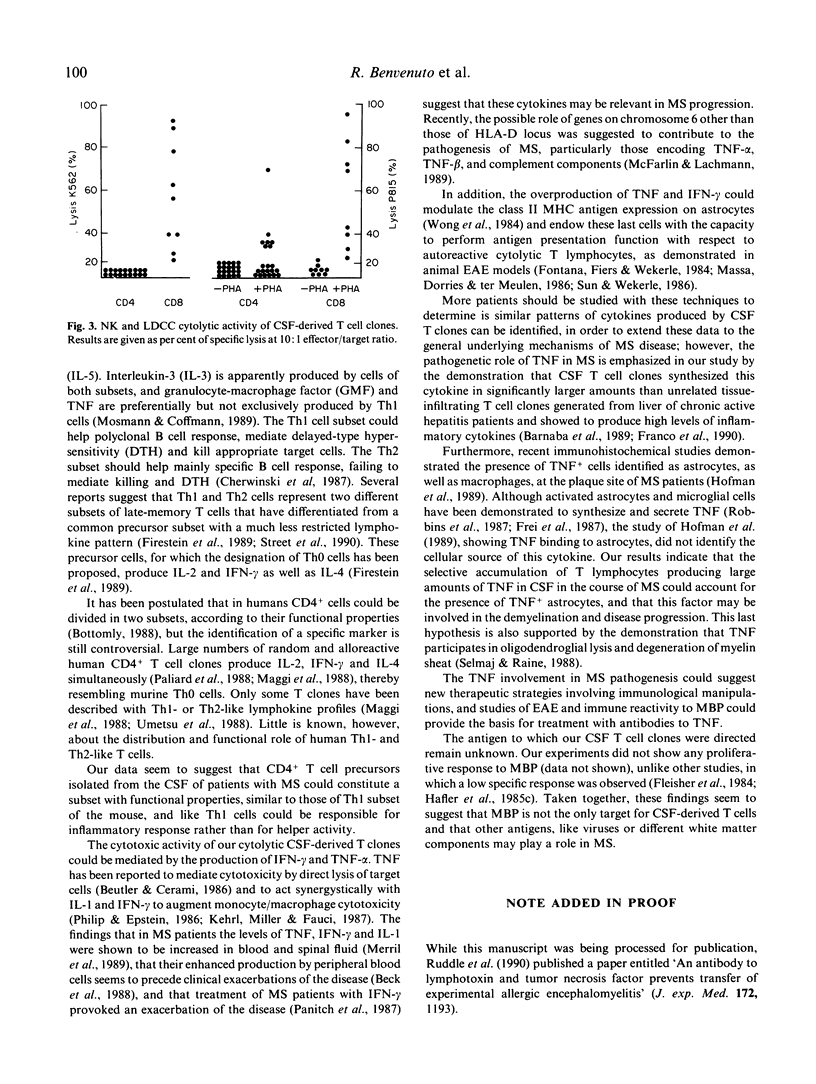
T cell clones derived from cerebrospinal fluid (CSF) of patients with multiple sclerosis (MS) were analysed for their ability to produce interferon-gamma (IFN-gamma), tumour necrosis factor-alpha (TNF-alpha), interleukin-2 (IL-2) and interleukin-4 (IL-4). The CSF-T clones were compared for their ability to produce cytokines with autologous peripheral T clones and with liver-infiltrating T cell clones from patients with chronic active hepatitis. IL-4 production was also compared with that by peripheral T clones derived from atopic patients. All the CSF-T clones (both CD4+ and CD8+) produced large amounts of IFN-gamma and particularly of TNF-alpha. These cytokines were synthesized in significantly larger amounts by CSF T clones than by reference clones. Moreover, they were capable of secreting IL-2, but not IL-4. We conclude that the CSF-CD4+ T clones could constitute a subset with functional properties similar to those of T helper 1 (Th1)inflammatory cells of the mouse; and that the large amounts of TNF produced by CSF T cell clones strongly suggest a significant role for this cytokine in MS immunopathogenesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acha-Orbea H., Mitchell D. J., Timmermann L., Wraith D. C., Tausch G. S., Waldor M. K., Zamvil S. S., McDevitt H. O., Steinman L. Limited heterogeneity of T cell receptors from lymphocytes mediating autoimmune encephalomyelitis allows specific immune intervention. Cell. 1988 Jul 15;54(2):263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- Antel J. P., Arnason B. G., Medof M. E. Suppressor cell function in multiple sclerosis: correlation with clinical disease activity. Ann Neurol. 1979 Apr;5(4):338–342. doi: 10.1002/ana.410050406. [DOI] [PubMed] [Google Scholar]
- Barnaba V., Franco A., Alberti A., Balsano C., Benvenuto R., Balsano F. Recognition of hepatitis B virus envelope proteins by liver-infiltrating T lymphocytes in chronic HBV infection. J Immunol. 1989 Oct 15;143(8):2650–2655. [PubMed] [Google Scholar]
- Beck J., Rondot P., Catinot L., Falcoff E., Kirchner H., Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol Scand. 1988 Oct;78(4):318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- Benveniste E. N., Sparacio S. M., Bethea J. R. Tumor necrosis factor-alpha enhances interferon-gamma-mediated class II antigen expression on astrocytes. J Neuroimmunol. 1989 Dec;25(2-3):209–219. doi: 10.1016/0165-5728(89)90139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. K., Vandenbark A. A., Jones R. E., Hashim G., Offner H. Selection of encephalitogenic rat T-lymphocyte clones recognizing an immunodominant epitope on myelin basic protein. J Neurosci Res. 1989 Feb;22(2):181–187. doi: 10.1002/jnr.490220211. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Roeder W. D., Laxer J. A., Townsend K. S., Weaver C. T., Hom J. T., Linton J., Torbett B. E., Glasebrook A. L. A new murine CD4+ T cell subset with an unrestricted cytokine profile. J Immunol. 1989 Jul 15;143(2):518–525. [PubMed] [Google Scholar]
- Fleischer B., Marquardt P., Poser S., Kreth H. W. Phenotypic markers and functional characteristics of T lymphocyte clones from cerebrospinal fluid in multiple sclerosis. J Neuroimmunol. 1984 Dec;7(2-3):151–162. doi: 10.1016/s0165-5728(84)80015-6. [DOI] [PubMed] [Google Scholar]
- Fontana A., Fierz W., Wekerle H. Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature. 1984 Jan 19;307(5948):273–276. doi: 10.1038/307273a0. [DOI] [PubMed] [Google Scholar]
- Franco A., Barnaba V., Ruberti G., Benvenuto R., Balsano C., Musca A. Liver-derived T cell clones in autoimmune chronic active hepatitis: accessory cell function of hepatocytes expressing class II major histocompatibility complex molecules. Clin Immunol Immunopathol. 1990 Mar;54(3):382–394. doi: 10.1016/0090-1229(90)90052-r. [DOI] [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Buchsbaum M., Johnson D., Weiner H. L. Phenotypic and functional analysis of T cells cloned directly from the blood and cerebrospinal fluid of patients with multiple sclerosis. Ann Neurol. 1985 Oct;18(4):451–458. doi: 10.1002/ana.410180407. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Buchsbaum M., Weiner H. L. Decreased autologous mixed lymphocyte reaction in multiple sclerosis. J Neuroimmunol. 1985 Oct;9(6):339–347. doi: 10.1016/s0165-5728(85)80034-5. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Duby A. D., Lee S. J., Benjamin D., Seidman J. G., Weiner H. L. Oligoclonal T lymphocytes in the cerebrospinal fluid of patients with multiple sclerosis. J Exp Med. 1988 Apr 1;167(4):1313–1322. doi: 10.1084/jem.167.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler D. A., Fox D. A., Manning M. E., Schlossman S. F., Reinherz E. L., Weiner H. L. In vivo activated T lymphocytes in the peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. N Engl J Med. 1985 May 30;312(22):1405–1411. doi: 10.1056/NEJM198505303122201. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Weiner H. L. MS: a CNS and systemic autoimmune disease. Immunol Today. 1989 Mar;10(3):104–107. doi: 10.1016/0167-5699(89)90236-3. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L. Defective autologous mixed lymphocyte reactivity in multiple sclerosis. Clin Exp Immunol. 1986 Apr;64(1):107–113. [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. L., Panitch H. S., Johnson K. P. Lymphocytes from multiple sclerosis patients produce elevated levels of gamma interferon in vitro. J Clin Immunol. 1985 Nov;5(6):386–389. doi: 10.1007/BF00915335. [DOI] [PubMed] [Google Scholar]
- Hofman F. M., Hinton D. R., Johnson K., Merrill J. E. Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med. 1989 Aug 1;170(2):607–612. doi: 10.1084/jem.170.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Miller A., Fauci A. S. Effect of tumor necrosis factor alpha on mitogen-activated human B cells. J Exp Med. 1987 Sep 1;166(3):786–791. doi: 10.1084/jem.166.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H., DeFreitas E. C., Harper M. E., Sandberg-Wollheim M., Sheremata W. A., Robert-Guroff M., Saxinger C. W., Feinberg M. B., Wong-Staal F., Gallo R. C. Multiple sclerosis and human T-cell lymphotropic retroviruses. Nature. 1985 Nov 14;318(6042):154–160. doi: 10.1038/318154a0. [DOI] [PubMed] [Google Scholar]
- Maggi E., Del Prete G., Macchia D., Parronchi P., Tiri A., Chrétien I., Ricci M., Romagnani S. Profiles of lymphokine activities and helper function for IgE in human T cell clones. Eur J Immunol. 1988 Jul;18(7):1045–1050. doi: 10.1002/eji.1830180712. [DOI] [PubMed] [Google Scholar]
- Massa P. T., Dörries R., ter Meulen V. Viral particles induce Ia antigen expression on astrocytes. Nature. 1986 Apr 10;320(6062):543–546. doi: 10.1038/320543a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin D. E., Lachmann P. J. Multiple sclerosis. Hopeful genes and immunology. Nature. 1989 Oct 26;341(6244):693–694. doi: 10.1038/341693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin D. E., McFarland H. F. Multiple sclerosis (first of two parts). N Engl J Med. 1982 Nov 4;307(19):1183–1188. doi: 10.1056/NEJM198211043071905. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Strom S. R., Ellison G. W., Myers L. W. In vitro study of mediators of inflammation in multiple sclerosis. J Clin Immunol. 1989 Mar;9(2):84–96. doi: 10.1007/BF00916935. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Hafler D. A., Weiner H. L., Letvin N. L., Hagan M., Daley J., Schlossman S. F. Selective loss of the suppressor-inducer T-cell subset in progressive multiple sclerosis. Analysis with anti-2H4 monoclonal antibody. N Engl J Med. 1987 Jan 8;316(2):67–72. doi: 10.1056/NEJM198701083160202. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Panitch H. S., Hirsch R. L., Haley A. S., Johnson K. P. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987 Apr 18;1(8538):893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Robbins D. S., Shirazi Y., Drysdale B. E., Lieberman A., Shin H. S., Shin M. L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987 Oct 15;139(8):2593–2597. [PubMed] [Google Scholar]
- Ruddle N. H., Bergman C. M., McGrath K. M., Lingenheld E. G., Grunnet M. L., Padula S. J., Clark R. B. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990 Oct 1;172(4):1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUMACHER G. A., BEEBE G., KIBLER R. F., KURLAND L. T., KURTZKE J. F., MCDOWELL F., NAGLER B., SIBLEY W. A., TOURTELLOTTE W. W., WILLMON T. L. PROBLEMS OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS: REPORT BY THE PANEL ON THE EVALUATION OF EXPERIMENTAL TRIALS OF THERAPY IN MULTIPLE SCLEROSIS. Ann N Y Acad Sci. 1965 Mar 31;122:552–568. doi: 10.1111/j.1749-6632.1965.tb20235.x. [DOI] [PubMed] [Google Scholar]
- Selmaj K. W., Raine C. S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988 Apr;23(4):339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Sgroi D., Cohen R. N., Lingenheld E. G., Strong M. K., Binder T., Goldschneider I., Greiner D., Grunnet M., Clark R. B. T cell lines derived from the spinal cords of mice with experimental allergic encephalomyelitis are self reactive. J Immunol. 1986 Sep 15;137(6):1850–1854. [PubMed] [Google Scholar]
- Street N. E., Schumacher J. H., Fong T. A., Bass H., Fiorentino D. F., Leverah J. A., Mosmann T. R. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990 Mar 1;144(5):1629–1639. [PubMed] [Google Scholar]
- Sun D., Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986 Mar 6;320(6057):70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- Traugott U., Lebon P. Interferon-gamma and Ia antigen are present on astrocytes in active chronic multiple sclerosis lesions. J Neurol Sci. 1988 Apr;84(2-3):257–264. doi: 10.1016/0022-510x(88)90130-x. [DOI] [PubMed] [Google Scholar]
- Umetsu D. T., Jabara H. H., DeKruyff R. H., Abbas A. K., Abrams J. S., Geha R. S. Functional heterogeneity among human inducer T cell clones. J Immunol. 1988 Jun 15;140(12):4211–4216. [PubMed] [Google Scholar]
- Urban J. L., Kumar V., Kono D. H., Gomez C., Horvath S. J., Clayton J., Ando D. G., Sercarz E. E., Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988 Aug 12;54(4):577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Walsh M. J., Tourtellotte W. W. Temporal invariance and clonal uniformity of brain and cerebrospinal IgG, IgA, and IgM in multiple sclerosis. J Exp Med. 1986 Jan 1;163(1):41–53. doi: 10.1084/jem.163.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Ota K., Endo N., Seidman J. G., Rosenzweig A., Weiner H. L., Hafler D. A. Shared human T cell receptor V beta usage to immunodominant regions of myelin basic protein. Science. 1990 May 25;248(4958):1016–1019. doi: 10.1126/science.1693015. [DOI] [PubMed] [Google Scholar]


