Abstract
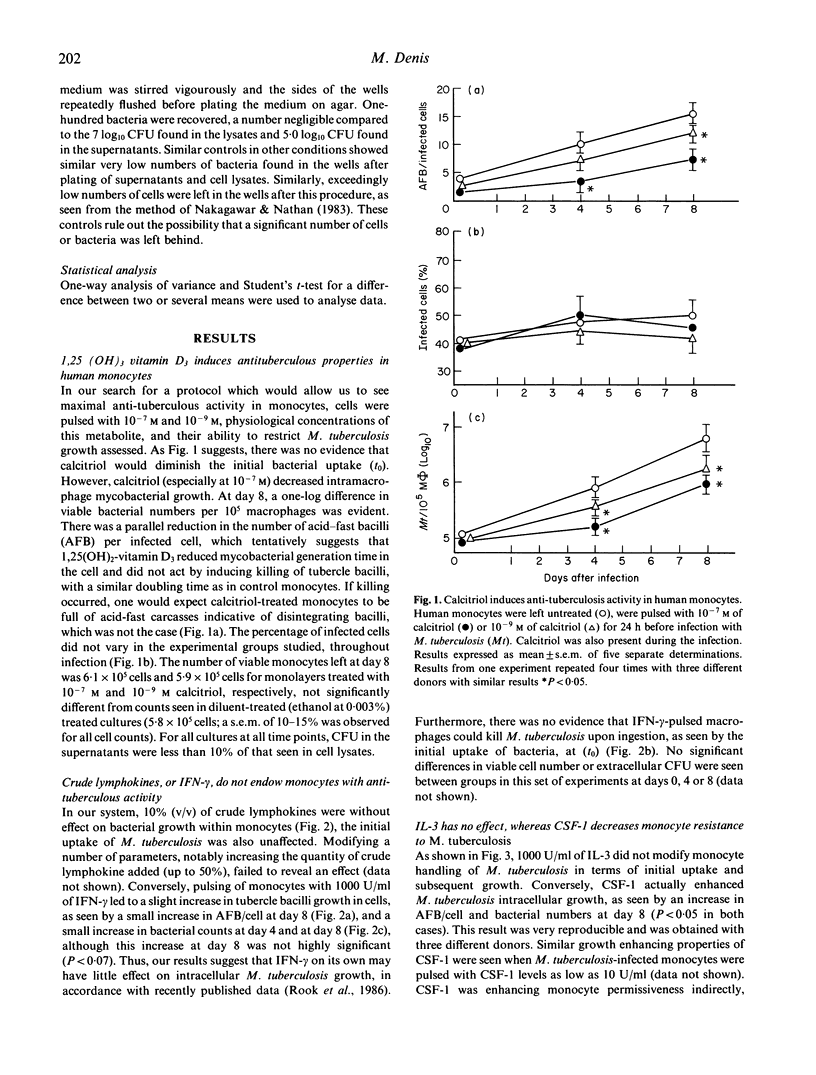
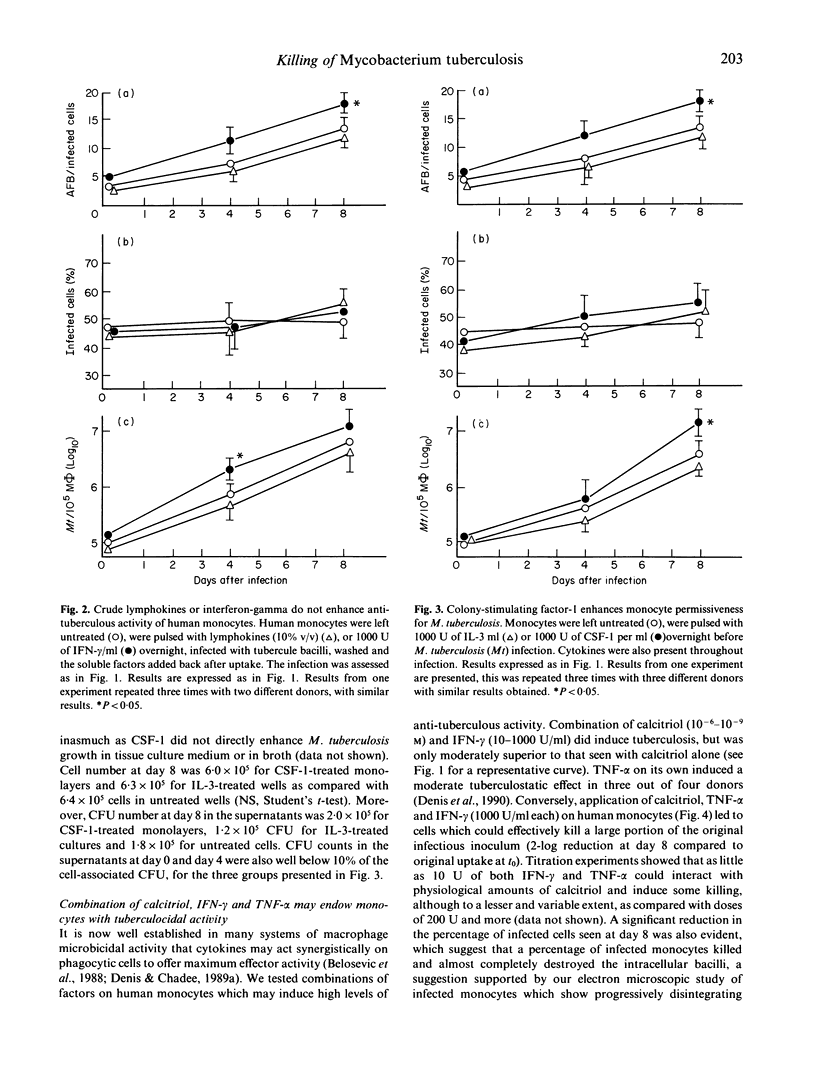
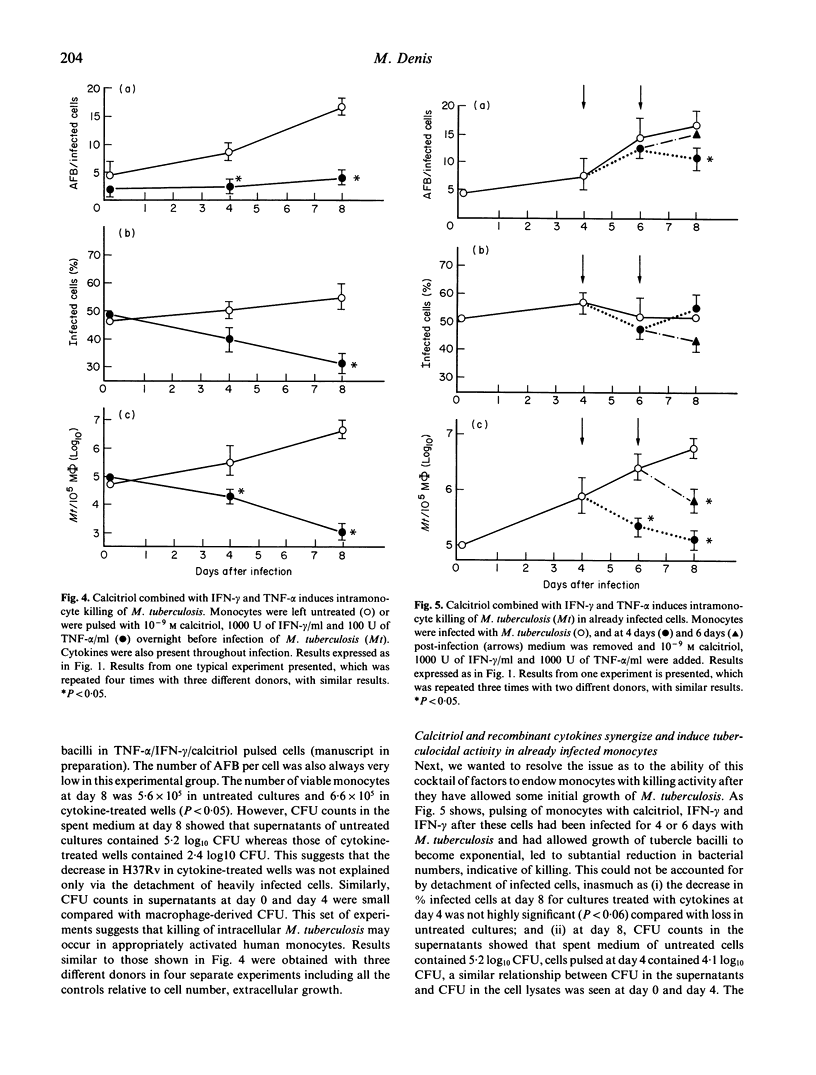
Human monocytes were isolated and their ability to harbour growth of virulent tubercle bacilli was assessed, in the presence or absence of various immunomodulators. Calcitriol (1,25(OH2), vitamin D3) alone, at doses of 10(-7)-10(-9) M endowed human monocytes with a significant ability to restrict intracellular growth of the tubercle bacilli. Crude immune lymphokines as well as recombinant interferon-gamma (IFN-gamma) endowed monocytes with no tuberculostatic activity. Similarly, other recombinant cytokines tested, notably colony-stimulating factor-1 (CSF-1), interleukin-1 (IL-1), interleukin-3 (IL-3) and interleukin-6 (IL-6) all failed to stimulate anti-tuberculous properties, and even increased growth of the tubercle bacilli in monocytes, in the case of CSF-1. Conversely, incubation of crude lymphokines in combination with calcitriol led to total stasis of the growth of M. tuberculosis. Experiments with recombinant cytokines and immunologically active vitamins showed that a combination of IFN-gamma tumour necrosis factor-alpha and calcitriol induced a significant amount of intramonocyte killing of M. tuberculosis. Addition of this cocktail of factors to already infected monocytes led to substantial killing of tubercle bacilli. These sets of experiments establish clearly that combinations of recombinant cytokines and vitamins may induce substantial intramonocyte killing of M. tuberculosis. The mechanism involved in this killing activity was not clarified.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belosevic M., Davis C. E., Meltzer M. S., Nacy C. A. Regulation of activated macrophage antimicrobial activities. Identification of lymphokines that cooperate with IFN-gamma for induction of resistance to infection. J Immunol. 1988 Aug 1;141(3):890–896. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S., Gupta S. 1,25 Dihydroxyvitamin D3-dependent inhibition of growth or killing of Mycobacterium avium complex in human macrophages is mediated by TNF and GM-CSF. Cell Immunol. 1990 May;127(2):432–441. doi: 10.1016/0008-8749(90)90144-g. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Catterall J. R., Sharma S. D., Remington J. S. Oxygen-independent killing by alveolar macrophages. J Exp Med. 1986 May 1;163(5):1113–1131. doi: 10.1084/jem.163.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M. In vivo vs. in vitro killing of virulent Mycobacterium tuberculosis. Res Microbiol. 1990 Feb;141(2):212–266. doi: 10.1016/0923-2508(90)90033-m. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Intracellular killing of mycobacteria. Res Microbiol. 1990 Feb;141(2):231–236. doi: 10.1016/0923-2508(90)90035-o. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., May M. H. Replication of lyophilized and cultured BCG in human macrophages. Am Rev Respir Dis. 1983 Oct;128(4):673–679. doi: 10.1164/arrd.1983.128.4.673. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Ross E. J., May M. H. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987 Dec;55(12):2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. D. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle. 1985 Dec;66(4):301–306. doi: 10.1016/0041-3879(85)90068-6. [DOI] [PubMed] [Google Scholar]
- Denis M., Chadee K. Cytokine activation of murine macrophages for in vitro killing of Entamoeba histolytica trophozoites. Infect Immun. 1989 Jun;57(6):1750–1756. doi: 10.1128/iai.57.6.1750-1756.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Chadee K. Human neutrophils activated by interferon-gamma and tumour necrosis factor-alpha kill Entamoeba histolytica trophozoites in vitro. J Leukoc Biol. 1989 Sep;46(3):270–274. doi: 10.1002/jlb.46.3.270. [DOI] [PubMed] [Google Scholar]
- Denis M., Ghadirian E. Granulocyte-macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunol Lett. 1990 Jun;24(3):203–206. doi: 10.1016/0165-2478(90)90049-v. [DOI] [PubMed] [Google Scholar]
- Douvas G. S., Looker D. L., Vatter A. E., Crowle A. J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985 Oct;50(1):1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J. Sources of variability in assays of the interaction of mycobacteria with mononuclear phagocytes: of mice and men. Res Microbiol. 1990 Feb;141(2):237–240. doi: 10.1016/0923-2508(90)90036-p. [DOI] [PubMed] [Google Scholar]
- Esparza I., Männel D., Ruppel A., Falk W., Krammer P. H. Interferon gamma and lymphotoxin or tumor necrosis factor act synergistically to induce macrophage killing of tumor cells and schistosomula of Schistosoma mansoni. J Exp Med. 1987 Aug 1;166(2):589–594. doi: 10.1084/jem.166.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Attempts to characterize the mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages. Infect Immun. 1988 Jun;56(6):1464–1469. doi: 10.1128/iai.56.6.1464-1469.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frendl G., Beller D. I. Regulation of macrophage activation by IL-3. I. IL-3 functions as a macrophage-activating factor with unique properties, inducing Ia and lymphocyte function-associated antigen-1 but not cytotoxicity. J Immunol. 1990 May 1;144(9):3392–3399. [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Greil J., Bodendorfer B., Röllinghoff M., Solbach W. Application of recombinant granulocyte-macrophage colony-stimulating factor has a detrimental effect in experimental murine leishmaniasis. Eur J Immunol. 1988 Oct;18(10):1527–1533. doi: 10.1002/eji.1830181009. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Mor N., Goren M. B., Crowle A. J. Enhancement of growth of Mycobacterium lepraemurium in macrophages by gamma interferon. Infect Immun. 1989 Aug;57(8):2586–2587. doi: 10.1128/iai.57.8.2586-2587.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci U S A. 1984 Feb;81(3):908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Stimulation of macrophage tumoricidal activity by the growth and differentiation factor CSF-1. Cell Immunol. 1987 Apr 1;105(2):270–279. doi: 10.1016/0008-8749(87)90076-1. [DOI] [PubMed] [Google Scholar]
- Rook G. A. Progress in the immunology of the mycobacterioses. Clin Exp Immunol. 1987 Jul;69(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Fraher L., Barker S., Karmali R., O'Riordan J., Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986 Jan;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]


