Abstract
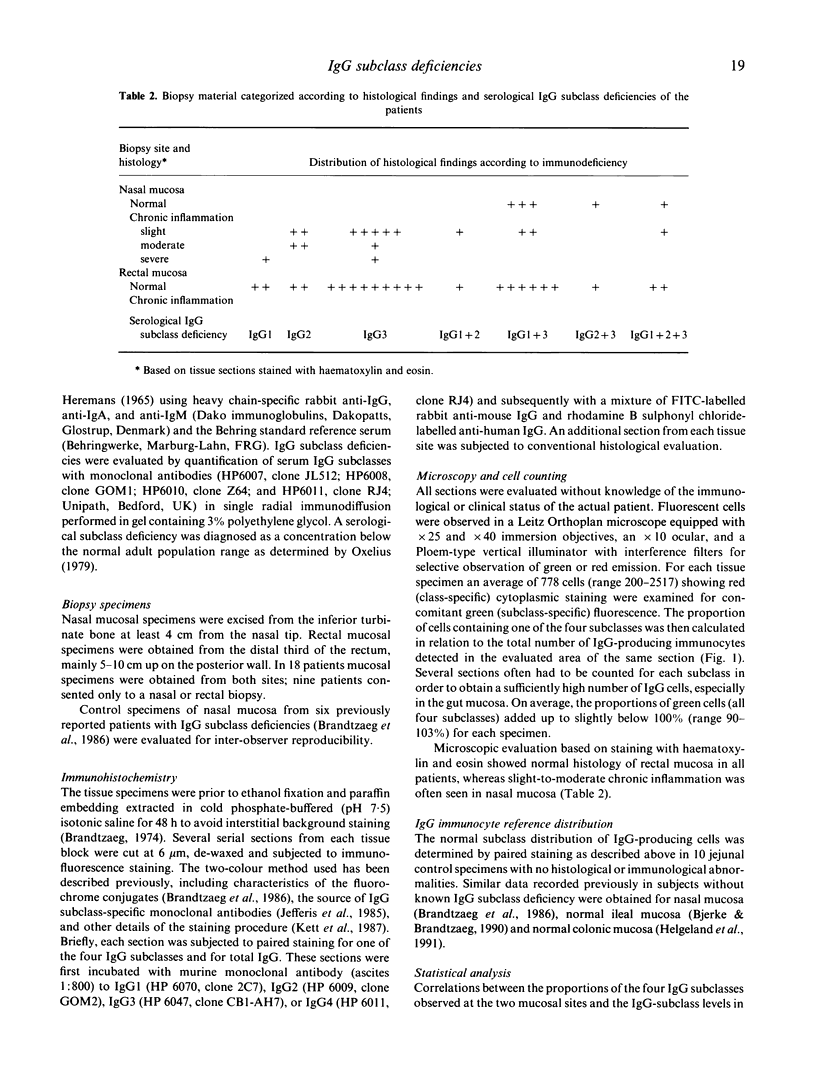
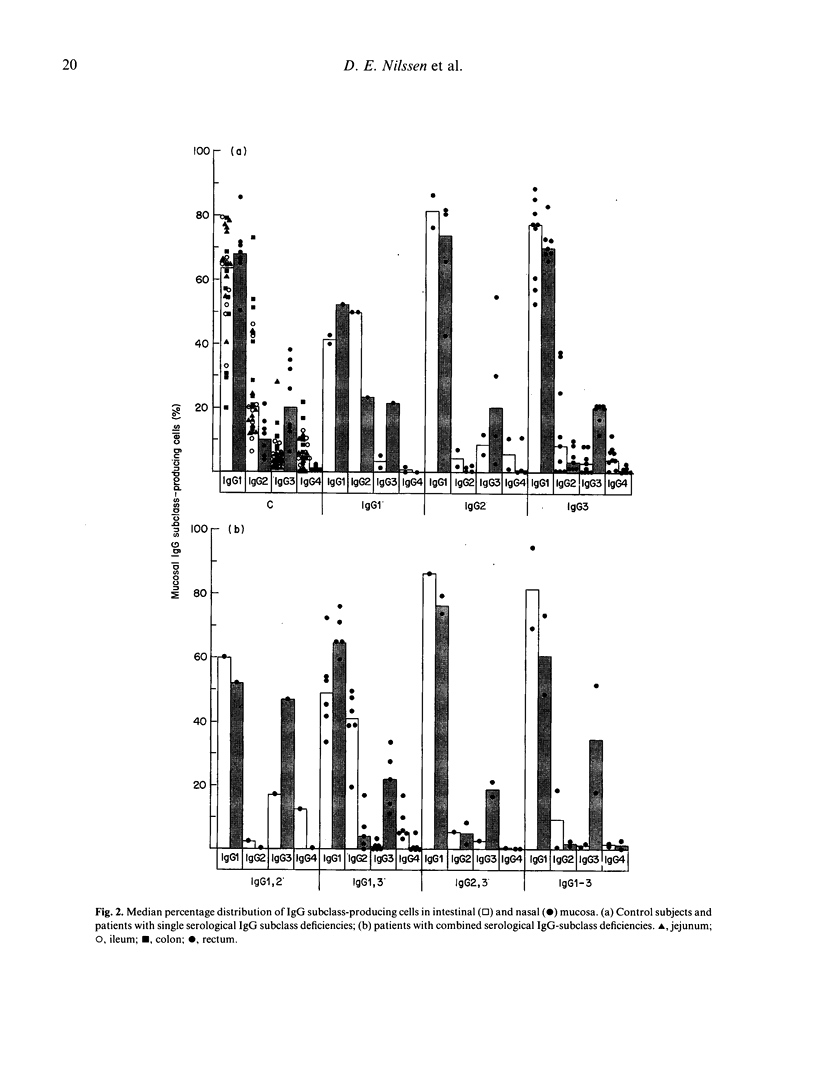
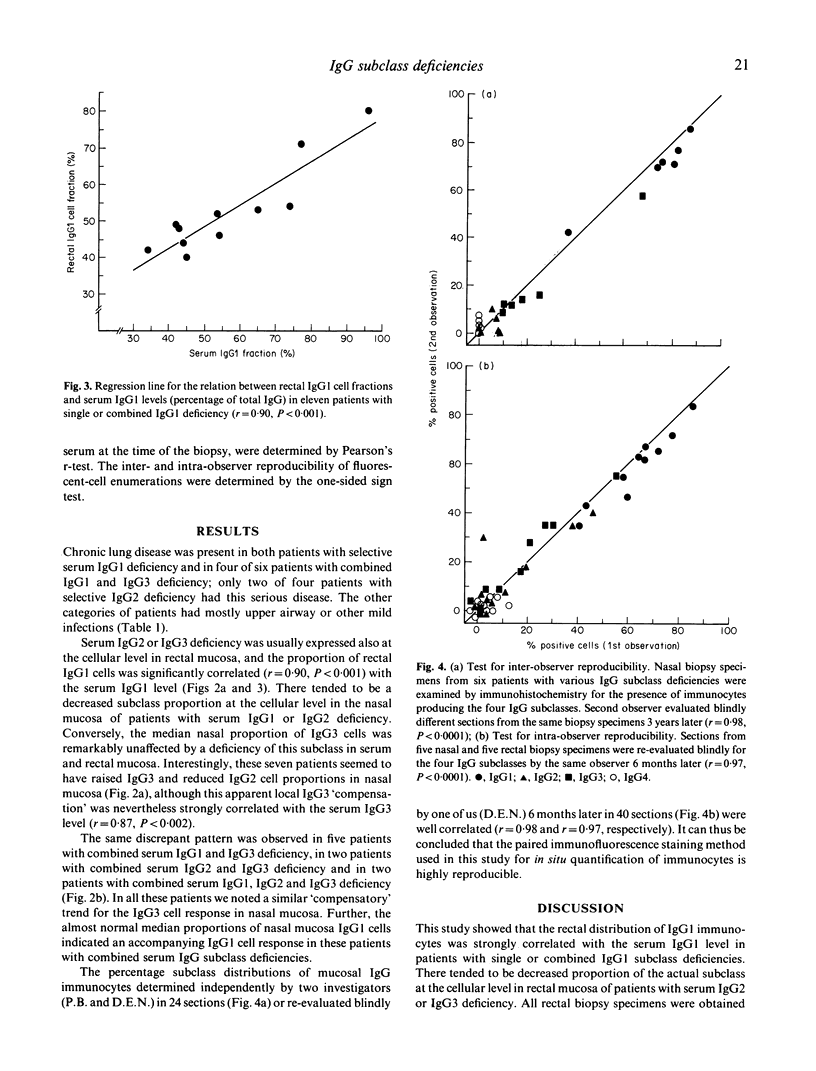
The subclass distribution of IgG-producing immunocytes was examined by immunohistochemistry in nasal and rectal mucosa of infection-prone patients with untreated IgG subclass deficiencies. Biopsy specimens from the two sites were obtained in 18 clinically and serologically well-characterized adult subjects; only a nasal or rectal sample was available from nine similar patients. Chronic lung disease was common in the patient groups with selective serum IgG1 deficiency and combined IgG1 and IgG3 deficiency, whereas the other categories of patients had mainly upper airway and other mild infections. Serum IgG2 or IgG3 deficiency was usually expressed also at the cellular level in rectal mucosa, and the proportion of rectal IgG1 cells was significantly correlated with the IgG1 level (r = 0.90, P less than 0.001). Likewise, there tended to be a decreased expression of the actual subclass at the cellular level in nasal mucosa of patients with serum IgG1 or IgG2 deficiency. Conversely, the median nasal proportion of IgG3 cells was remarkably unaffected by a deficiency of this subclass in serum and rectal mucosa. Interestingly, these patients rather tended to have raised IgG3 and reduced IgG2 cell proportions in their nasal mucosa, although this apparent local IgG3 compensation was nevertheless strongly correlated with the serum IgG3 level (r = 0.87, P less than 0.002). These disparities may reflect different antigenic and mitogenic exposure of the two tissue sites; for example, a persistent protein bombardment of the nasal mucosa that could conceivably override locally a B cell maturation defect. The possible clinical consequences of such variable mucosal expression of IgG subclass deficiencies remain to be studied.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjerke K., Brandtzaeg P. Terminally differentiated human intestinal B cells. IgA and IgG subclass-producing immunocytes in the distal ileum, including Peyer's patches, compared with lymph nodes and palatine tonsils. Scand J Immunol. 1990 Aug;32(2):61–67. doi: 10.1111/j.1365-3083.1990.tb02894.x. [DOI] [PubMed] [Google Scholar]
- Björkander J., Bengtsson U., Oxelius V. A., Hanson L. A. Symptoms in patients with lowered levels of IgG subclasses, with or without IgA deficiency, and effects of immunoglobulin prophylaxis. Monogr Allergy. 1986;20:157–163. [PubMed] [Google Scholar]
- Brandtzaeg P., Gjeruldsen S. T., Korsrud F., Baklien K., Berdal P., Ek J. The human secretory immune system shows striking heterogeneity with regard to involvement of J chain-positive IgD immunocytes. J Immunol. 1979 Feb;122(2):503–510. [PubMed] [Google Scholar]
- Brandtzaeg P., Karlsson G., Hansson G., Petruson B., Björkander J., Hanson L. A. The clinical condition of IgA-deficient patients is related to the proportion of IgD- and IgM-producing cells in their nasal mucosa. Clin Exp Immunol. 1987 Mar;67(3):626–636. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Kett K., Rognum T. O., Söderström R., Björkander J., Söderström T., Petrusson B., Hanson L. A. Distribution of mucosal IgA and IgG subclass-producing immunocytes and alterations in various disorders. Monogr Allergy. 1986;20:179–194. [PubMed] [Google Scholar]
- Brandtzaeg P., Valnes K., Scott H., Rognum T. O., Bjerke K., Baklien K. The human gastrointestinal secretory immune system in health and disease. Scand J Gastroenterol Suppl. 1985;114:17–38. doi: 10.3109/00365528509093765. [DOI] [PubMed] [Google Scholar]
- CRABBE P. A., CARBONARA A. O., HEREMANS J. F. THE NORMAL HUMAN INTESTINAL MUCOSA AS A MAJOR SOURCE OF PLASMA CELLS CONTAINING GAMMA-A-IMMUNOGLOBULIN. Lab Invest. 1965 Mar;14:235–248. [PubMed] [Google Scholar]
- Eddie D. S., Schulkind M. L., Robbins J. B. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium "O" antibodies from rabbit intestinal fluid and colostrum. J Immunol. 1971 Jan;106(1):181–190. [PubMed] [Google Scholar]
- Eidelman S. Intestinal lesions in immune deficiency. Hum Pathol. 1976 Jul;7(4):427–434. doi: 10.1016/s0046-8177(76)80056-1. [DOI] [PubMed] [Google Scholar]
- FAZEKAS DE ST GROTH S., DONNELLEY M., GRAHAM D. M. Studies in experimental immunology of influenza. VIII. Pathotopic adjuvants. Aust J Exp Biol Med Sci. 1951 Sep;29(5):323–327. doi: 10.1038/icb.1951.39. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982 Dec 23;300(5894):709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- Hammarström L., Granström M., Oxelius V., Persson M. A., Smith C. I. IgG subclass distribution of antibodies against S. aureus teichoic acid and alpha-toxin in normal and immunodeficient donors. Clin Exp Immunol. 1984 Mar;55(3):593–601. [PMC free article] [PubMed] [Google Scholar]
- Hammarström L., Mellstedt H., Persson M. A., Smith C. I., Ahre A. IgA subclass distribution in paraproteinemias: suggestion of an IgG-IgA subclass switch pattern. Acta Pathol Microbiol Immunol Scand C. 1984 Aug;92(4):207–211. doi: 10.1111/j.1699-0463.1984.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Haneberg B., Endresen C. Fragments of immunoglobulins in human faeces. Acta Pathol Microbiol Scand C. 1976 Feb;84(1):31–36. doi: 10.1111/j.1699-0463.1976.tb03596.x. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Reimer C. B., Skvaril F., de Lange G., Ling N. R., Lowe J., Walker M. R., Phillips D. J., Aloisio C. H., Wells T. W. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10(3-4):223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Kett K., Rognum T. O., Brandtzaeg P. Mucosal subclass distribution of immunoglobulin G-producing cells is different in ulcerative colitis and Crohn's disease of the colon. Gastroenterology. 1987 Nov;93(5):919–924. doi: 10.1016/0016-5085(87)90552-x. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mygind N., Weeke B., Ullman S. Quantitative determination of immunoglobulins in nasal secretion. Int Arch Allergy Appl Immunol. 1975;49(1-2):99–107. doi: 10.1159/000231383. [DOI] [PubMed] [Google Scholar]
- Newhouse M., Sanchis J., Bienenstock J. Lung defense mechanisms (second of two parts). N Engl J Med. 1976 Nov 4;295(19):1045–1052. doi: 10.1056/NEJM197611042951905. [DOI] [PubMed] [Google Scholar]
- Oxelius V. A., Hanson L. A., Björkander J., Hammarström L., Sjöholm A. IgG3 deficiency: common in obstructive lung disease. Hereditary in families with immunodeficiency and autoimmune disease. Monogr Allergy. 1986;20:106–115. [PubMed] [Google Scholar]
- Oxelius V. A. IgG subclass levels in infancy and childhood. Acta Paediatr Scand. 1979 Jan;68(1):23–27. doi: 10.1111/j.1651-2227.1979.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Rognum T. O., Kett K., Fausa O., Bengtsson U., Kilander A., Scott H., Gaarder P. I., Brandtzaeg P. Raised number of jejunal IgG2-producing cells in untreated adult coeliac disease compared with food allergy. Gut. 1989 Nov;30(11):1574–1580. doi: 10.1136/gut.30.11.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schur P. H., Borel H., Gelfand E. W., Alper C. A., Rosen F. S. Selective gamma-g globulin deficiencies in patients with recurrent pyogenic infections. N Engl J Med. 1970 Sep 17;283(12):631–634. doi: 10.1056/NEJM197009172831205. [DOI] [PubMed] [Google Scholar]
- Scott M. G., Nahm M. H., Macke K., Nash G. S., Bertovich M. J., MacDermott R. P. Spontaneous secretion of IgG subclasses by intestinal mononuclear cells: differences between ulcerative colitis, Crohn's disease, and controls. Clin Exp Immunol. 1986 Oct;66(1):209–215. [PMC free article] [PubMed] [Google Scholar]
- Shakib F., Stanworth D. R. Human IgG subclasses in health and disease. (A review). Part I. Ric Clin Lab. 1980 Jul-Sep;10(3):463–479. doi: 10.1007/BF02938793. [DOI] [PubMed] [Google Scholar]
- Stokes C. R., Soothill J. F., Turner M. W. Immune exclusion is a function of IgA. Nature. 1975 Jun 26;255(5511):745–746. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- Söderström T., Söderström R., Bengtsson U., Björkander J., Hellstrand K., Holm J., Hanson L. A. Clinical and immunological evaluation of patients low in single or multiple IgG subclasses. Monogr Allergy. 1986;20:135–142. [PubMed] [Google Scholar]
- Söderström T., Söderström R., Hanson L. A. Immunoglobulin G subclasses in immunodeficiency. Ann Clin Res. 1987;19(4):280–284. [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Valnes K., Brandtzaeg P. Subclass distribution of mucosal IgG-producing cells in gastritis. Gut. 1989 Mar;30(3):322–326. doi: 10.1136/gut.30.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]


