Abstract
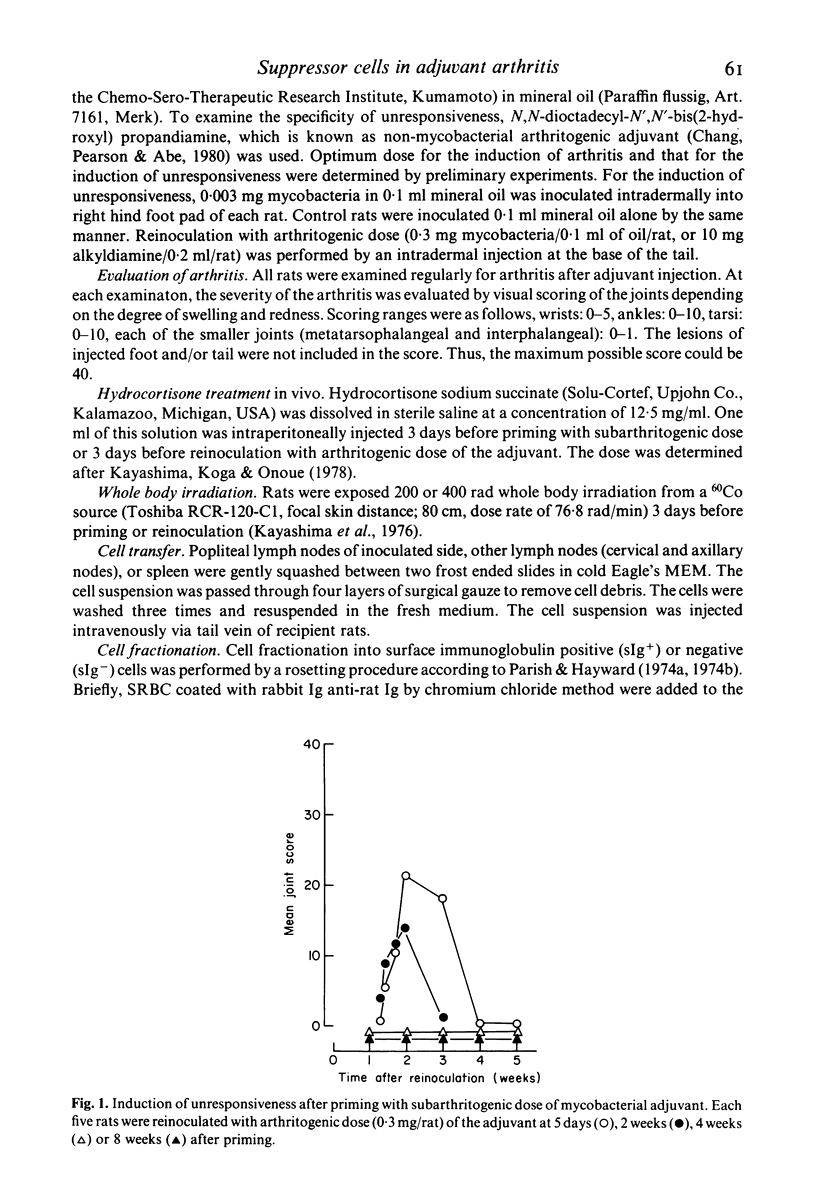
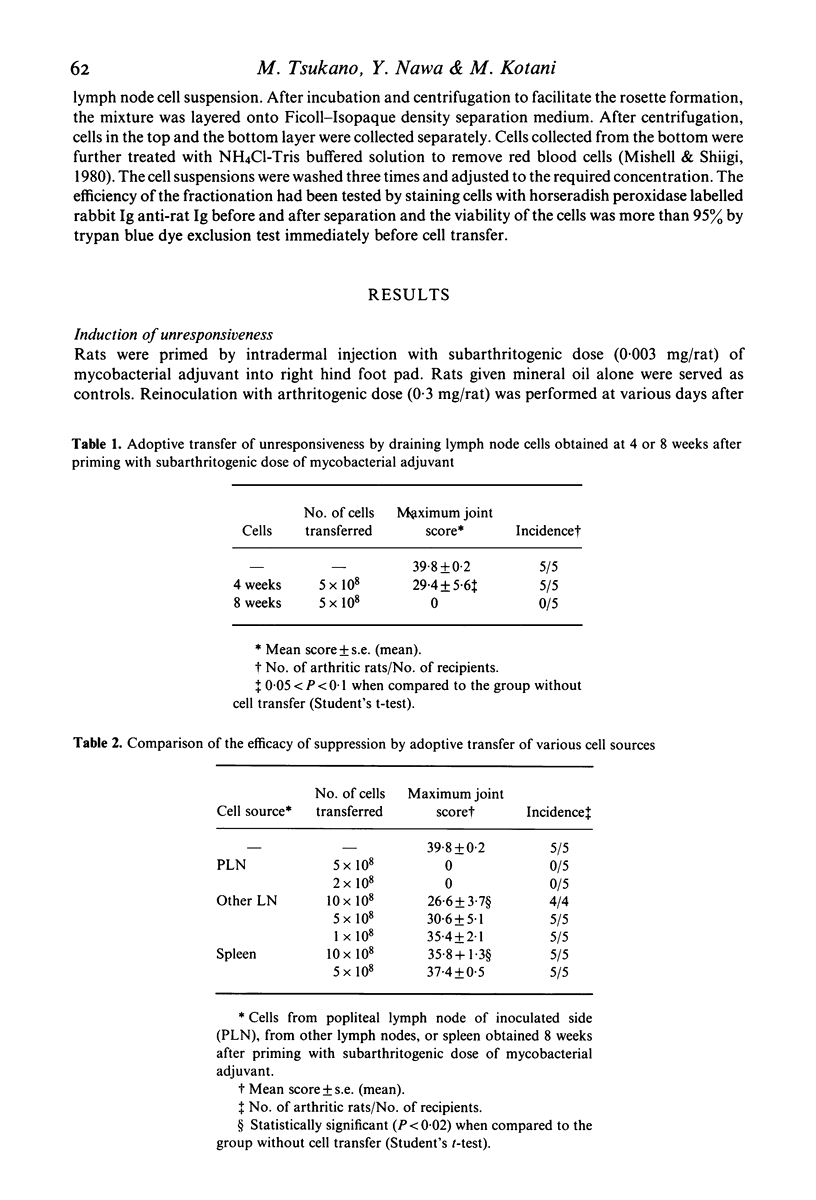
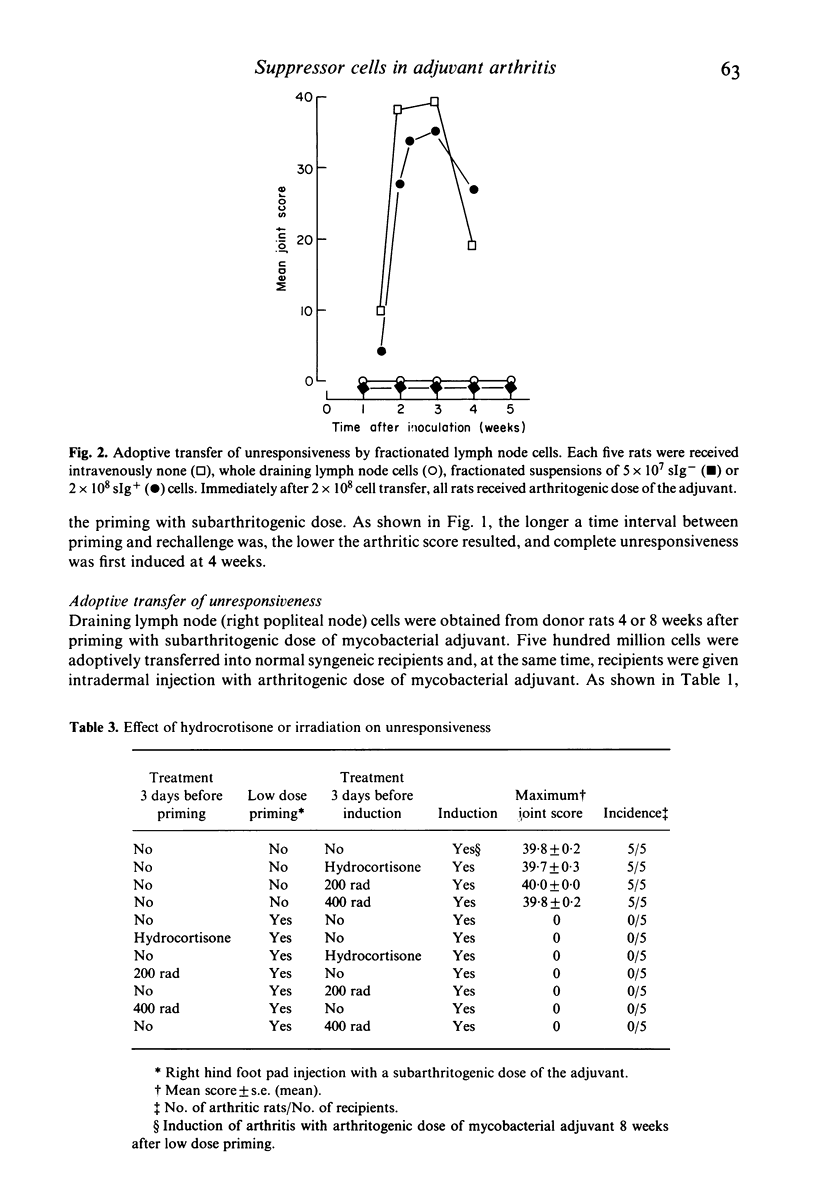
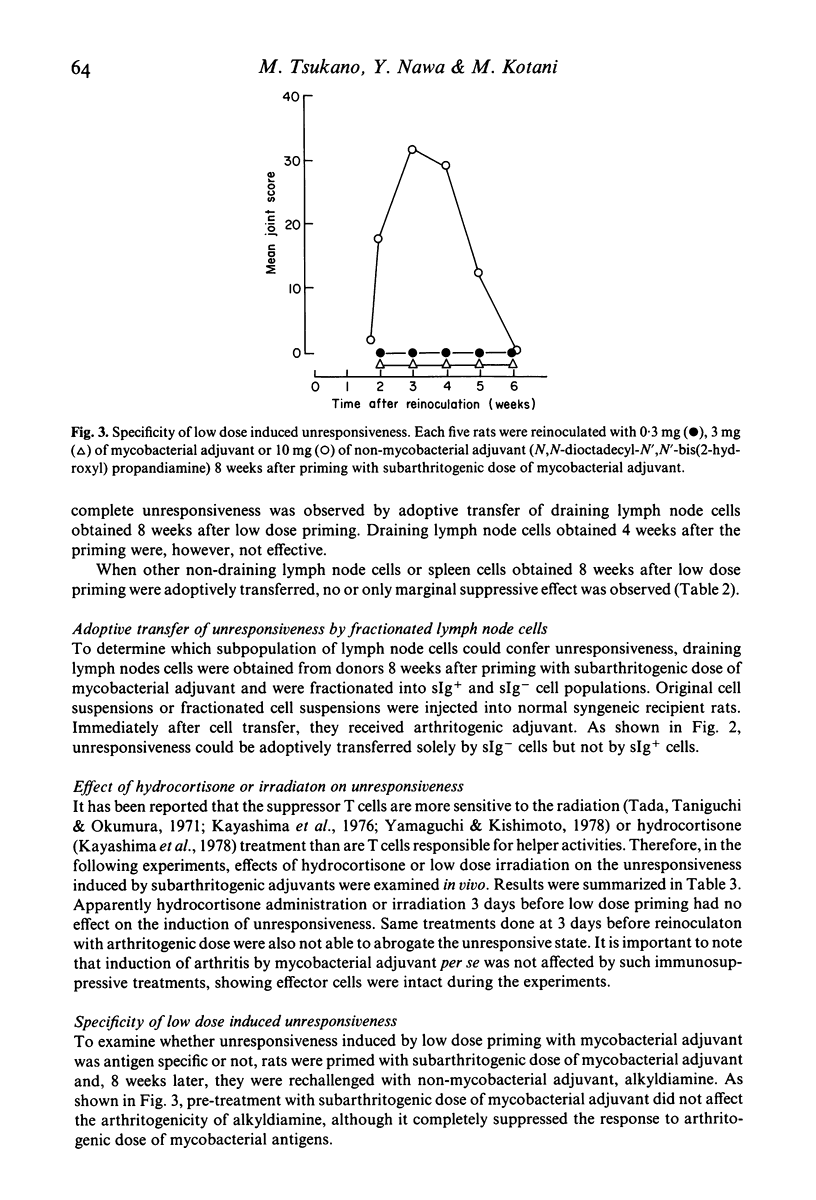
Characterization of suppressor cells in adjuvant arthritis was performed by using highly susceptible DA strain rats. The results showed that suppressor cells were induced after a single inoculation of subarthritogenic dose of mycobacterial adjuvant. Relatively long incubation period was required for the induction of suppressor cells. Such cells were predominated in the draining lymph node and, after fractionation, only sIg- population was effective in conferring unresponsiveness. In vivo irradiation or hydrocortisone treatment suggested that low dose induced suppressor cells were resistant against such immunosuppressive treatments. In addition, by using alkyldiamine as a non-mycobacterial arthritogenic adjuvant, it was suggested that unresponsiveness induced by low dose priming with mycobacterial adjuvant was antigen specific.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang Y. H., Pearson C. M., Abe C. Adjuvant polyarthritis. IV. Induction by a synthetic adjuvant: immunologic, histopathologic, and other studies. Arthritis Rheum. 1980 Jan;23(1):62–71. doi: 10.1002/art.1780230111. [DOI] [PubMed] [Google Scholar]
- Eugui E. M., Houssay R. H. Passive transfer of unresponsiveness by lymph node cells. Studies on adjuvant disease. Immunology. 1975 Apr;28(4):703–710. [PMC free article] [PubMed] [Google Scholar]
- Kayashima K., Koga T., Onoue K. Role of T lymphocytes in adjuvant arthritis. I. Evidence for the regulatory function of thymus-derived cells in the induction of the disease. J Immunol. 1976 Nov;117(5 PT2):1878–1882. [PubMed] [Google Scholar]
- Kayashima K., Koga T., Onoue K. Role of T lymphocytes in adjuvant arthritis. II. Different subpopulations of T lymphocytes functioning in the development of the disease. J Immunol. 1978 Apr;120(4):1127–1131. [PubMed] [Google Scholar]
- Mackenzie A. R., Pick C. R., Sibley P. R., White B. P. Suppression of rat adjuvant disease by cyclophosphamide pretreatment: evidence for an antibody mediated component in the pathogenesis of the disease. Clin Exp Immunol. 1978 Apr;32(1):86–96. [PMC free article] [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M., WOOD F. D. PASSIVE TRANSFER OF ADJUVANT ARTHRITIS BY LYMPH NODE OR SPLEEN CELLS. J Exp Med. 1964 Oct 1;120:547–560. doi: 10.1084/jem.120.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., Okumura K. Regulation of homocytotropic antibody formation in the rat. II. Effect of X-irradiation. J Immunol. 1971 Apr;106(4):1012–1018. [PubMed] [Google Scholar]
- WAKSMAN B. H., PEARSON C. M., SHARP J. T. Studies of arthritis and other lesions induced in rats by injection of mycobacterial adjuvant. II. Evidence that the disease is a disseminated immunologic response to exogenous antigen. J Immunol. 1960 Oct;85:403–417. [PubMed] [Google Scholar]
- Watson S. R., Collins F. M. Development of suppressor T cells in mice heavily infected with mycobacteria. Immunology. 1980 Mar;39(3):367–373. [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Kishimoto S. Distinction between suppressors of the delayed-type hypersensitivity and the humoral response to sheep erythrocytes. Immunology. 1978 Nov;35(5):721–731. [PMC free article] [PubMed] [Google Scholar]


