Abstract
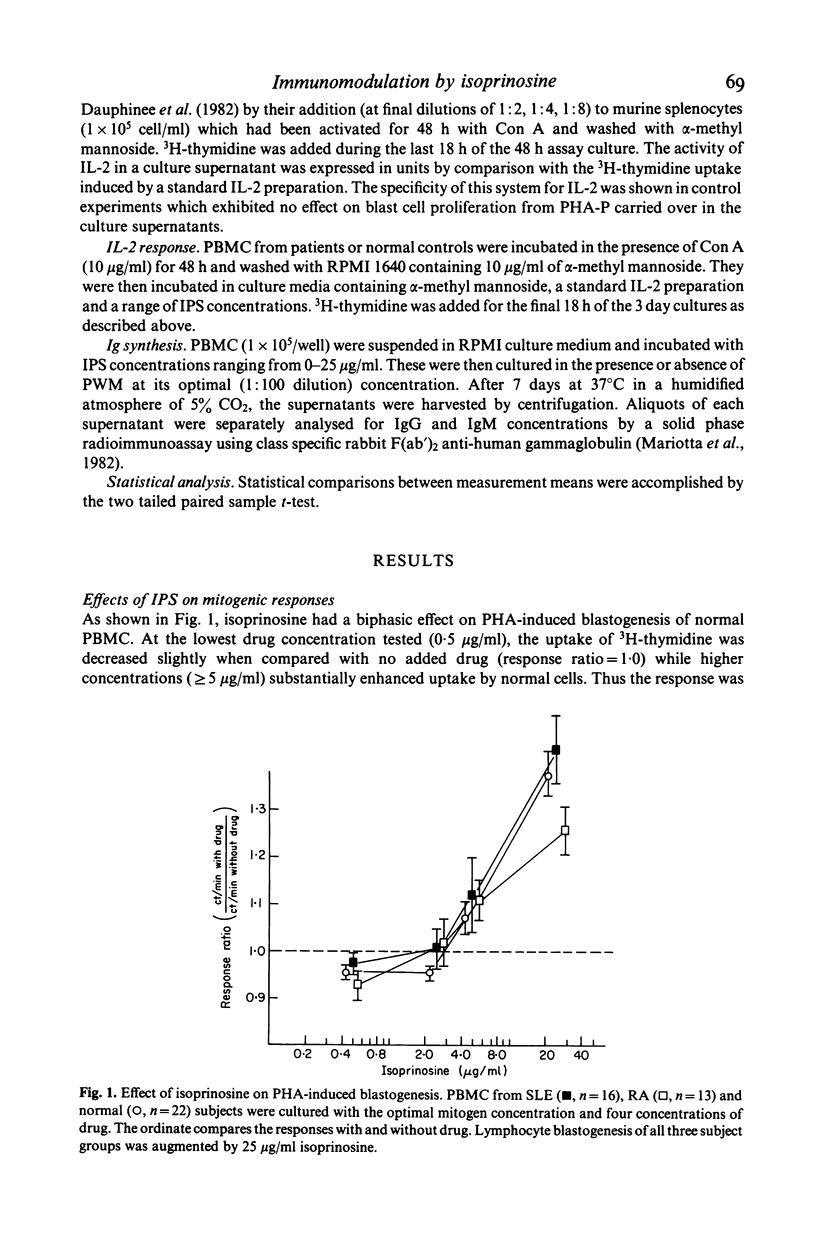
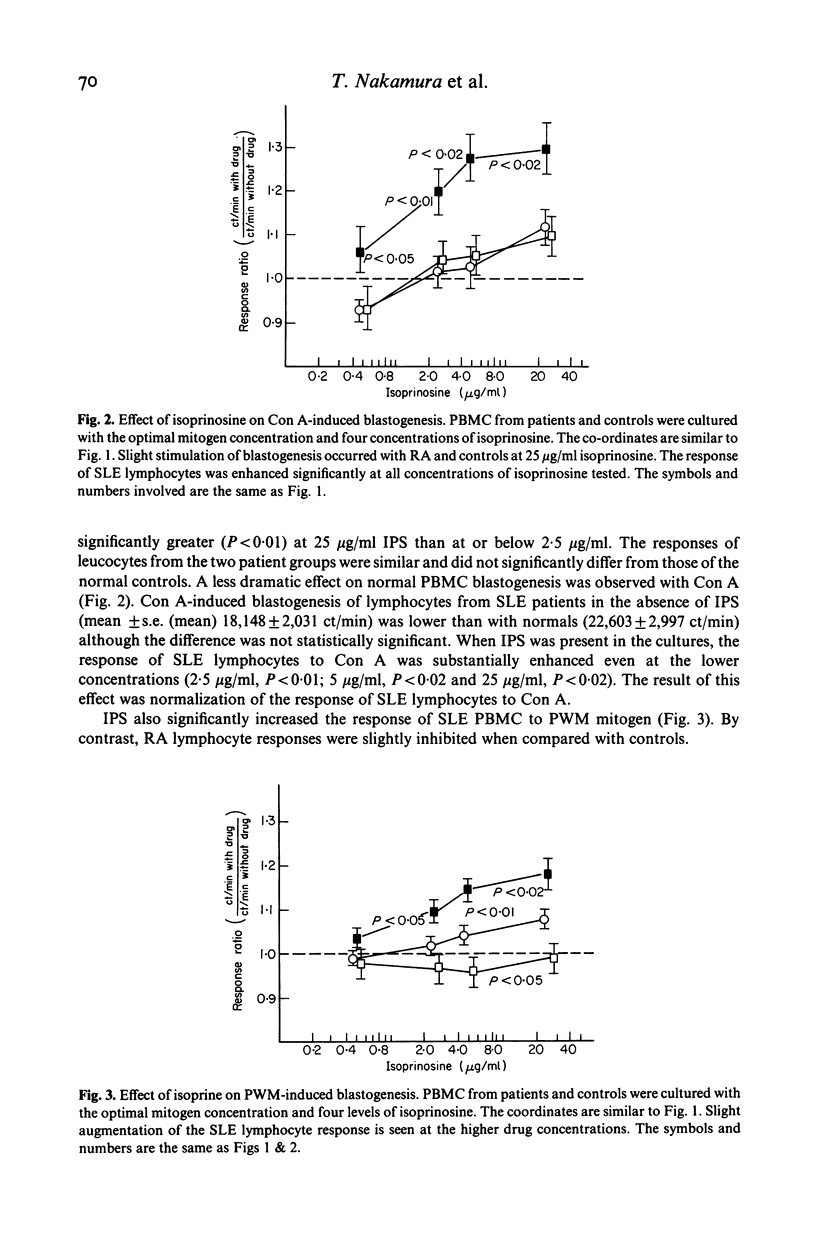
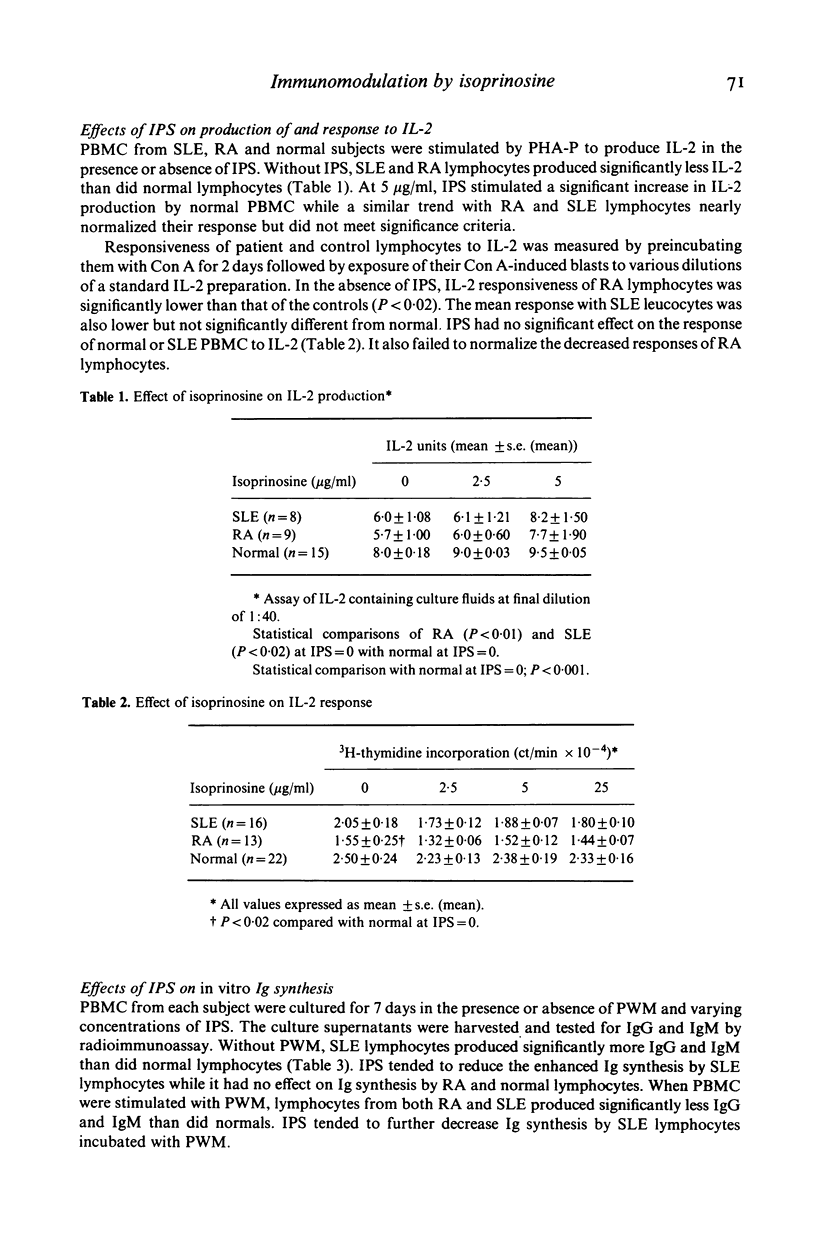
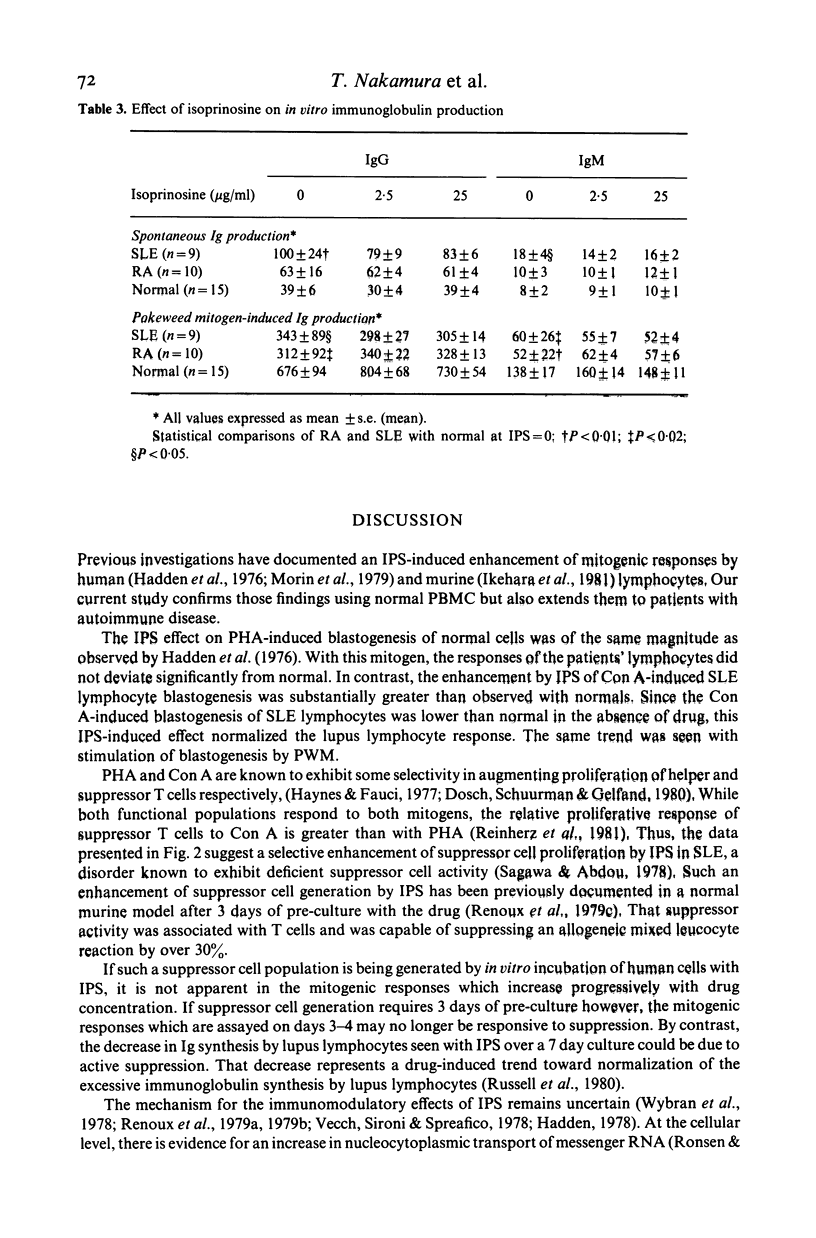
Isoprinosine (IPS) is a new anti-viral agent which appears to have immunomodulatory activities which include its ability to enhance the in vitro blastogenic responses of normal lymphocytes to mitogens. The present study compares the effects of IPS on the in vitro immune functions of peripheral blood mononuclear cells (PBMC) from systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) patients with its effects on PBMC from normal controls. Each mitogen (Con A, PHA or PWM) was used at its optimal concentration with a range of IPS concentrations (0-25 micrograms/ml). PHA-induced blastogenesis by PBMC from all three groups was enhanced by IPS at or above 5 micrograms/ml. The Con A-induced responses of SLE lymphocytes were significantly enhanced over controls by IPS (P less than 0.02 at 5 micrograms/ml) while those of RA lymphocytes were not. IPS had little effect on PWM-induced blastogenesis by RA lymphocytes but did enhance the blastogenic responses of SLE lymphocytes (P less than 0.01 at 5 micrograms/ml). In contrast, the characteristically high immunoglobulin synthesis by SLE lymphocytes was decreased by IPS. The mechanism responsible for these effects is not known but IL-2 production by patient lymphocytes in vitro which was low for both RA (P less than 0.01) and SLE (P less than 0.02) increased significantly (P less than 0.05) when SLE lymphocytes were cultured with IPS. These data identify IPS as an agent for the study of aberrant immune regulation in autoimmune diseases and suggest that it may have potential therapeutic value in SLE.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnard G. D., Yasaka K., Jacobson D. Ligand-activated T cell growth factor-induced proliferation: absorption of T cell growth factor by activated T cells. J Immunol. 1979 Dec;123(6):2704–2708. [PubMed] [Google Scholar]
- Cerutti I., Chany C., Schlumberger J. F. Isoprinosine increases the antitumor action of interferon. Int J Immunopharmacol. 1979;1(1):59–63. doi: 10.1016/0192-0561(79)90031-6. [DOI] [PubMed] [Google Scholar]
- Dauphinée M. J., Kipper S. B., Wofsy D., Talal N. Interleukin 2 deficiency is a common feature of autoimmune mice. J Immunol. 1981 Dec;127(6):2483–2487. [PubMed] [Google Scholar]
- Dosch H. M., Schuurman R. K., Gelfand E. W. Polyclonal activation of human lymphocytes in vitro-II. Reappraisal of T and B cell-specific mitogens. J Immunol. 1980 Aug;125(2):827–832. [PubMed] [Google Scholar]
- Dosch H. M., Shore A. Hypothesis: the role of interleukins in lymphopoiesis - important in autoimmune disease? J Rheumatol. 1982 May-Jun;9(3):353–358. [PubMed] [Google Scholar]
- Gillis S., Kozak R., Durante M., Weksler M. E. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981 Apr;67(4):937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg T., Glasky A. J. Inosiplex: an immunomodulation model for the treatment of viral disease. Ann N Y Acad Sci. 1977 Mar 4;284:128–138. doi: 10.1111/j.1749-6632.1977.tb21944.x. [DOI] [PubMed] [Google Scholar]
- Haddad F. S., Risk W. S. Isoprinosine treatment in 18 patients with subacute sclerosing panencephalitis: a controlled study. Ann Neurol. 1980 Feb;7(2):185–188. doi: 10.1002/ana.410070216. [DOI] [PubMed] [Google Scholar]
- Hadden J. W. Effects of isoprinosine, levamisole, muramyl dipeptide, and SM1213 on lymphocyte and macrophage function in vitro. Cancer Treat Rep. 1978 Nov;62(11):1981–1985. [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Coffey R. G. Isoprinosine augmentation of phytohemagglutinin-induced lymphocyte proliferation. Infect Immun. 1976 Feb;13(2):382–387. doi: 10.1128/iai.13.2.382-387.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Fauci A. S. Activation of human B lymphocytes. III. Concanavalin A-induced generation of suppressor cells of the plaque-forming cell response of normal human B lymphocytes. J Immunol. 1977 Jun;118(6):2281–2287. [PubMed] [Google Scholar]
- Larsson E. L. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J Immunol. 1981 Apr;126(4):1323–1326. [PubMed] [Google Scholar]
- López-Botet M., Fontán G., Garcia Rodriguez M. C., de Landázuri M. O. Relationship between IL 2 synthesis and the proliferative response to PHA in different primary immunodeficiencies. J Immunol. 1982 Feb;128(2):679–683. [PubMed] [Google Scholar]
- Mariotti S., Oger J. J., Fragu P., Antel J. P., Kuo H. H., DeGroot L. J. A new solid-phase radioimmunoassay to measure IgG secreted by cultured human lymphocytes. J Immunol Methods. 1980;35(3-4):189–199. doi: 10.1016/0022-1759(80)90246-x. [DOI] [PubMed] [Google Scholar]
- Morin A., Griscelli C., Daguillard F. Effets de l'isoprinosine sur l'activation des lymphocytes humains in vitro. Ann Immunol (Paris) 1979 Jul-Aug;130C(4):541–551. [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Reinherz E. L., Penta A. C., Hussey R. E., Schlossman S. F. A rapid method for separating functionally intact human T lymphocytes with monoclonal antibodies. Clin Immunol Immunopathol. 1981 Nov;21(2):257–266. doi: 10.1016/0090-1229(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Renoux G., Renoux M., Degenne D. Suppressor cell activity after isoprinosine treatment of lymphocytes from normal mice. Int J Immunopharmacol. 1979;1(3):239–241. doi: 10.1016/0192-0561(79)90048-1. [DOI] [PubMed] [Google Scholar]
- Renoux G., Renoux M., Guillaumin J. M., Gouzien C. Differentiation and regulation of lymphocyte populations: evidence for immunopotentiator-induced T cell recruitment. J Immunopharmacol. 1979;1(3):415–422. doi: 10.3109/08923977909026383. [DOI] [PubMed] [Google Scholar]
- Renoux G., Renoux M., Guillaumin J. M. Isoprinosine as an immunopotentiator. J Immunopharmacol. 1979;1(3):337–356. doi: 10.3109/08923977909026379. [DOI] [PubMed] [Google Scholar]
- Ronsen B., Gordon P. Methisoprinol enhancement of nucleocytoplasmic transport of putative messenger RNA in rat liver. its relation to host defense against virus infection. Biochem Pharmacol. 1976 Mar 15;25(6):707–715. doi: 10.1016/0006-2952(76)90248-3. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Conn D. L., McKenna C. H., Stobo J. D. Augmentation of immunoglobulin production in connective tissue disorders. Clin Immunol Immunopathol. 1980 Jun;16(2):221–232. doi: 10.1016/0090-1229(80)90206-8. [DOI] [PubMed] [Google Scholar]
- Sagawa A., Abdou N. I. Suppressor-cell dysfunction in systemic lupus erythematosus. Cells involved and in vitro correction. J Clin Invest. 1978 Oct;62(4):789–796. doi: 10.1172/JCI109190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergiescu D., Cerutti I., Kahan A., Piatier D., Efthymiou E. Isoprinosine delays the early appearance of autoimmunity in NZB/NZW F1 mice treated with interferon. Clin Exp Immunol. 1981 Jan;43(1):36–45. [PMC free article] [PubMed] [Google Scholar]
- Soto A. J., Hall T. S., Reed S. E. Trial of the antiviral action of isoprinosine against rhinovirus infection of volunteers. Antimicrob Agents Chemother. 1973 Mar;3(3):332–334. doi: 10.1128/aac.3.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi A., Sironi M., Spreafico F. Preliminary characterization in mice of the effect of isoprinosine on the immune system. Cancer Treat Rep. 1978 Nov;62(11):1975–1979. [PubMed] [Google Scholar]
- Waldman R. H., Ganguly R. Therapeutic efficacy of inosiplex (Isoprinosine) in rhinovirus infection. Ann N Y Acad Sci. 1977 Mar 4;284:153–160. doi: 10.1111/j.1749-6632.1977.tb21946.x. [DOI] [PubMed] [Google Scholar]
- Wybran J., Famaey J. P., Appelboom T. Inosiplex: a novel treatment in rheumatoid arthritis? J Rheumatol. 1981 Jul-Aug;8(4):643–646. [PubMed] [Google Scholar]
- Wybran J., Govaerts A., Appelboom T. Inosiplex, a stimulating agent for normal human T cells and human leukocytes. J Immunol. 1978 Sep;121(3):1184–1187. [PubMed] [Google Scholar]


