Abstract
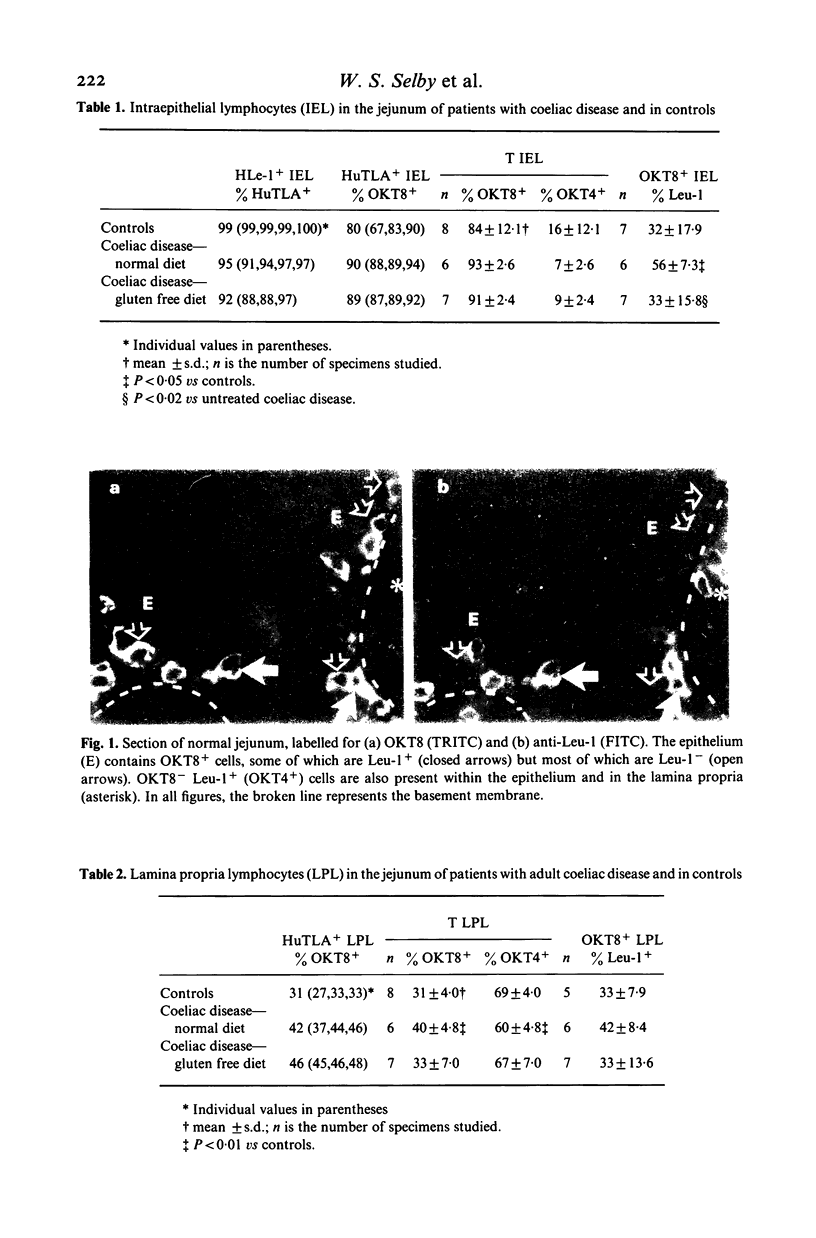
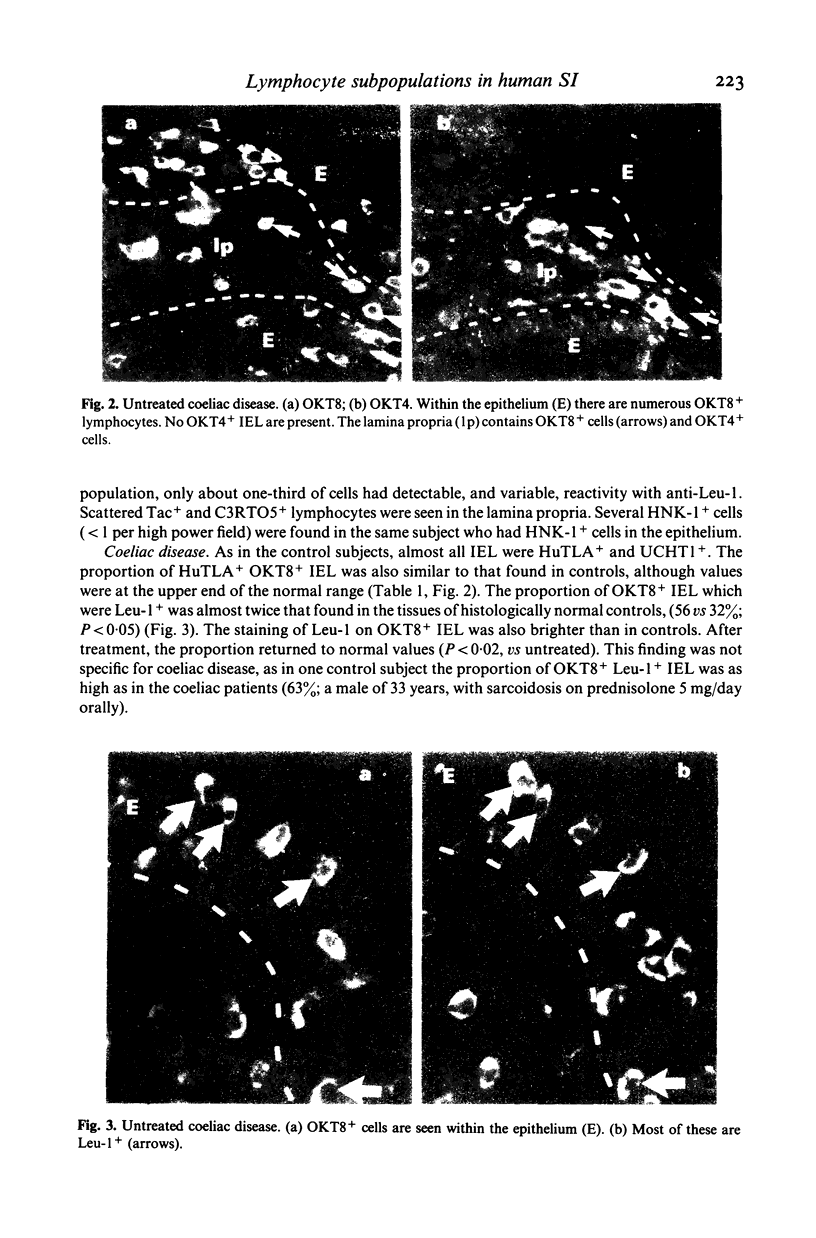
Lymphocyte subpopulations in human small intestinal mucosa have been studied using an immunofluorescence technique on tissue sections. In the normal intestine, the majority of intraepithelial lymphocytes (IEL) were of suppressor-cytotoxic phenotype (HuTLA+ UCHTI+ OKT8+ OKT4-; 84%). Only one-third of these OKT8+IEL reacted with anti-Leu-1, and antibody directed towards a 67,000 dalton antigen found on peripheral blood T cells. IEL failed to express the activation antigen, Tac, and also lacked detectable C3b receptor (C3RTO5-). The remaining T IEL, as well as the predominant lamina propria T lymphocytes (LPL), were OKT4+ OKT8-, helper type T cells. Most of the lamina propria OKT8+ cells were also Leu-1-. In patients with adult coeliac disease, the proportions of OKT8+ and OKT4+ lymphocytes in the epithelium were not altered. However, the proportion of OKT8+ Leu-1+TIEL was significantly increased (56 vs 32%; P less than 0.02). IEL were also HLA-DR-, Tac- and C3RTO5-. The proportion of OKT8+ cells in the lamina propria was slightly, but significantly, increased (40 vs 32%; P less than 0.005). Mucosal findings in treated patients did not differ from normal. Lymphocytes with the phenotype of natural killer cells (HNK-1) were rarely found in normal or diseased mucosa. No alterations in the proportions of circulating T lymphocytes or their subsets were found in patients with coeliac disease. These findings illustrate the heterogeneity of lymphocyte subpopulations in normal and in diseased small intestinal mucosa. The changes found in adult coeliac disease may reflect the increased traffic of IEL into the epithelium.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Arnaud-Battandier F., Bundy B. M., O'Neill M., Bienenstock J., Nelson D. L. Cytotoxic activities of gut mucosal lymphoid cells in guinea pigs. J Immunol. 1978 Sep;121(3):1059–1065. [PubMed] [Google Scholar]
- Benacerraf B., Germain R. N. A single major pathway of T-lymphocyte interactions in antigen-specific immune suppression. Scand J Immunol. 1981;13(1):1–10. doi: 10.1111/j.1365-3083.1981.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Beverley P. C., Woody J., Dunkley M., Feldmann M., McKenzie I. Separation of suppressor and killer T cells by surgace phenotype. Nature. 1976 Aug 5;262(5568):495–497. doi: 10.1038/262495a0. [DOI] [PubMed] [Google Scholar]
- Callard R. E., Smith C. M., Worman C., Linch D., Cawley J. C., Beverley P. C. Unusual phenotype and function of an expanded subpopulation of T cells in patients with haemopoietic disorders. Clin Exp Immunol. 1981 Mar;43(3):497–505. [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Boyse E. Regulation of the immune response by T-cell subclasses. Contemp Top Immunobiol. 1977;7:47–67. doi: 10.1007/978-1-4684-3054-7_2. [DOI] [PubMed] [Google Scholar]
- Chiba M., Bartnik W., ReMine S. G., Thayer W. R., Shorter R. G. Human colonic intraepithelial and lamina proprial lymphocytes: cytotoxicity in vitro and the potential effects of the isolation method on their functional properties. Gut. 1981 Mar;22(3):177–186. doi: 10.1136/gut.22.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. D., Parrott D. M. Cytotoxic T cells in small intestine epithelial, lamina propria and lung lymphocytes. Immunology. 1981 Oct;44(2):367–371. [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. A., Martin P. J., Kamoun M., Torok-Storb B., Newman W., Nowinski R. C., Thomas E. D. Monoclonal antibodies recognizing human T cells: potential role for preventing graft-versus-host reactions following allogeneic marrow transplantation. Transplant Proc. 1981 Mar;13(1 Pt 2):1133–1137. [PubMed] [Google Scholar]
- Jackson R. A., Morris M. A., Haynes B. F., Eisenbarth G. S. Increased circulating Ia-antigen-bearing T cells in type I diabetes mellitus. N Engl J Med. 1982 Apr 1;306(13):785–788. doi: 10.1056/NEJM198204013061305. [DOI] [PubMed] [Google Scholar]
- Janossy G., Bollum F. J., Bradstock K. F., McMichael A., Rapson N., Greaves M. F. Terminal transferase-positive human bone marrow cells exhibit the antigenic phenotype of common acute lymphoblastic leukemia. J Immunol. 1979 Oct;123(4):1525–1529. [PubMed] [Google Scholar]
- Janossy G., Tidman N., Papageorgiou E. S., Kung P. C., Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981 Apr;126(4):1608–1613. [PubMed] [Google Scholar]
- Ledbetter J. A., Evans R. L., Lipinski M., Cunningham-Rundles C., Good R. A., Herzenberg L. A. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man. J Exp Med. 1981 Feb 1;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyscom N., Brueton M. J. Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology. 1982 Apr;45(4):775–783. [PMC free article] [PubMed] [Google Scholar]
- Marsh M. N. Studies of intestinal lymphoid tissue. III. Quantitative analyses of epithelial lymphocytes in the small intestine of human control subjects and of patients with celiac sprue. Gastroenterology. 1980 Sep;79(3):481–492. [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Siadak A. W., Nowinski R. C. Monoclonal antibodies recognizing normal human T lymphocytes and malignant human B lymphocytes: a comparative study. J Immunol. 1981 Nov;127(5):1920–1923. [PubMed] [Google Scholar]
- McMichael A. J., Pilch J. R., Galfré G., Mason D. Y., Fabre J. W., Milstein C. A human thymocyte antigen defined by a hybrid myeloma monoclonal antibody. Eur J Immunol. 1979 Mar;9(3):205–210. doi: 10.1002/eji.1830090307. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Goldstein G., Jewell D. P. T lymphocyte subsets in human intestinal mucosa: the distribution and relationship to MHC-derived antigens. Clin Exp Immunol. 1981 Jun;44(3):453–458. [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Jewell D. P. Immunohistological characterisation of intraepithelial lymphocytes of the human gastrointestinal tract. Gut. 1981 Mar;22(3):169–176. doi: 10.1136/gut.22.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliabue A., Luini W., Soldateschi D., Boraschi D. Natural killer activity of gut mucosal lymphoid cells in mice. Eur J Immunol. 1981 Nov;11(11):919–922. doi: 10.1002/eji.1830111112. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]


