Abstract
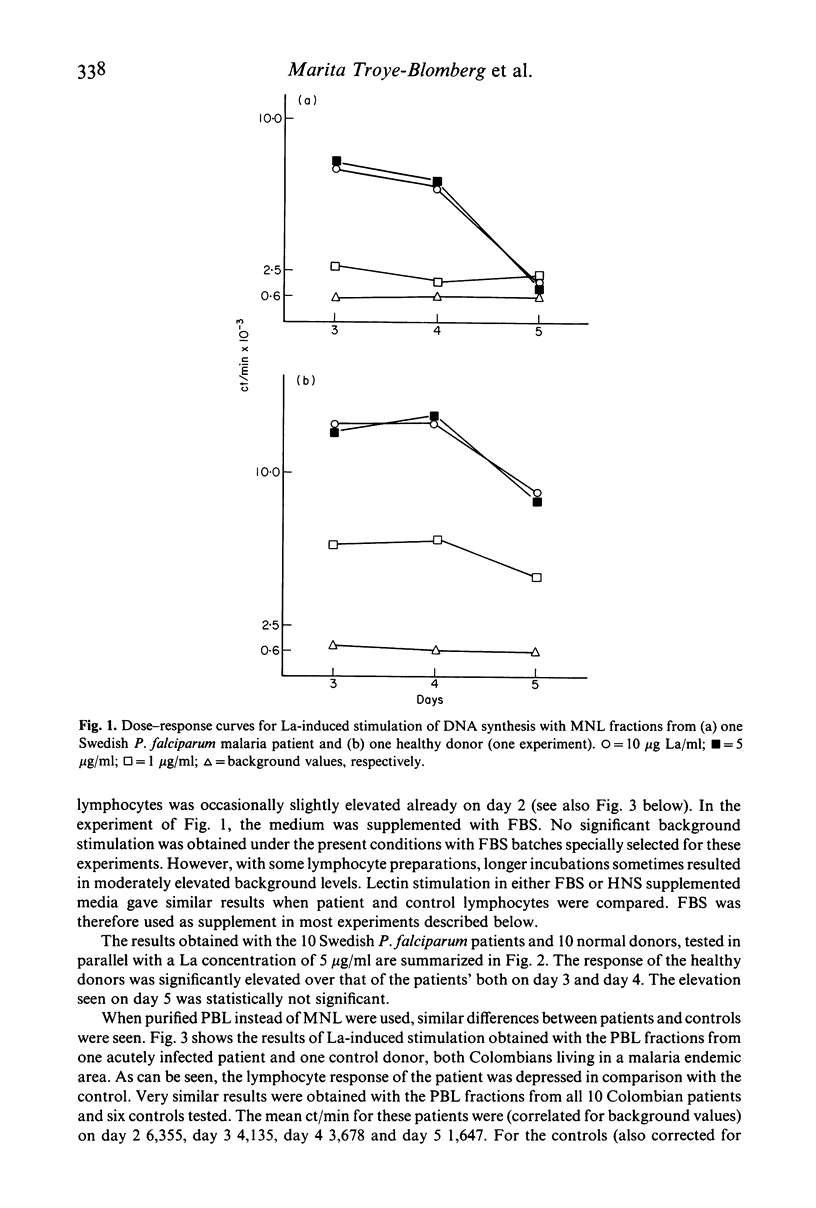
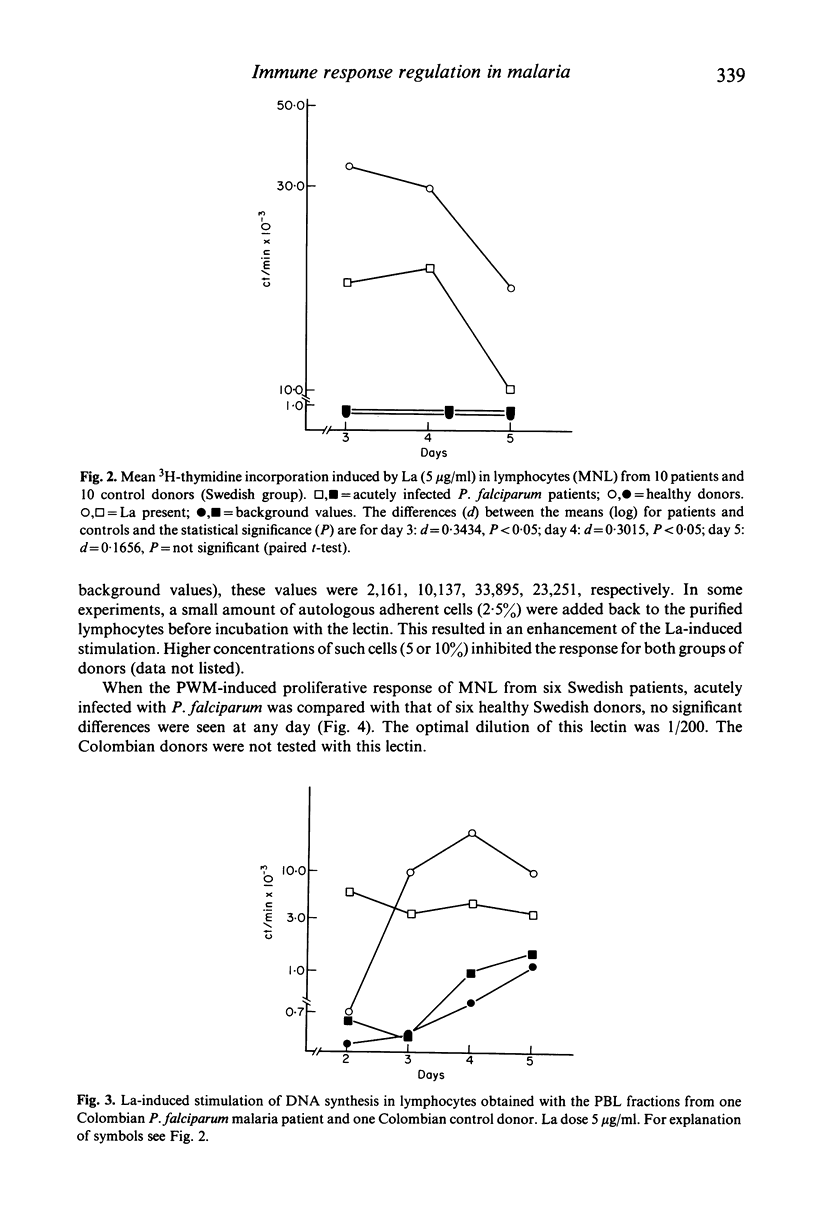
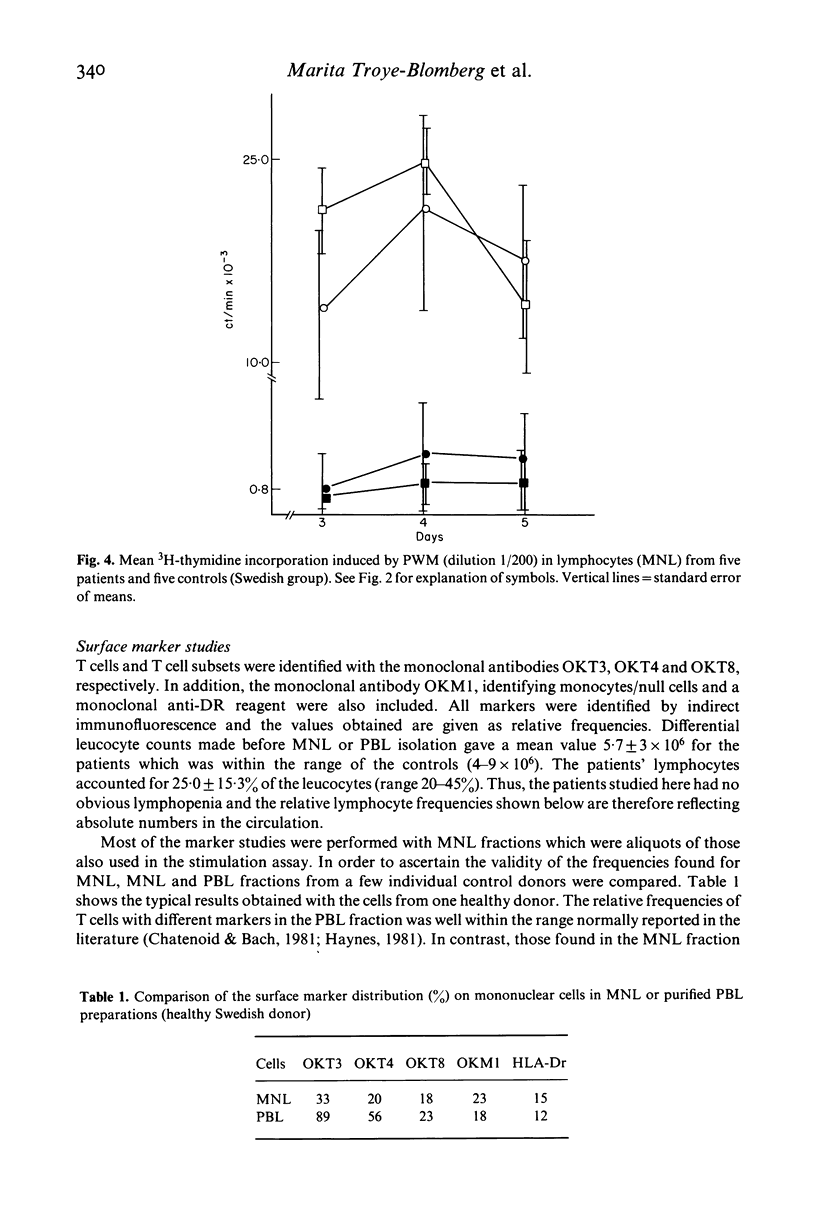
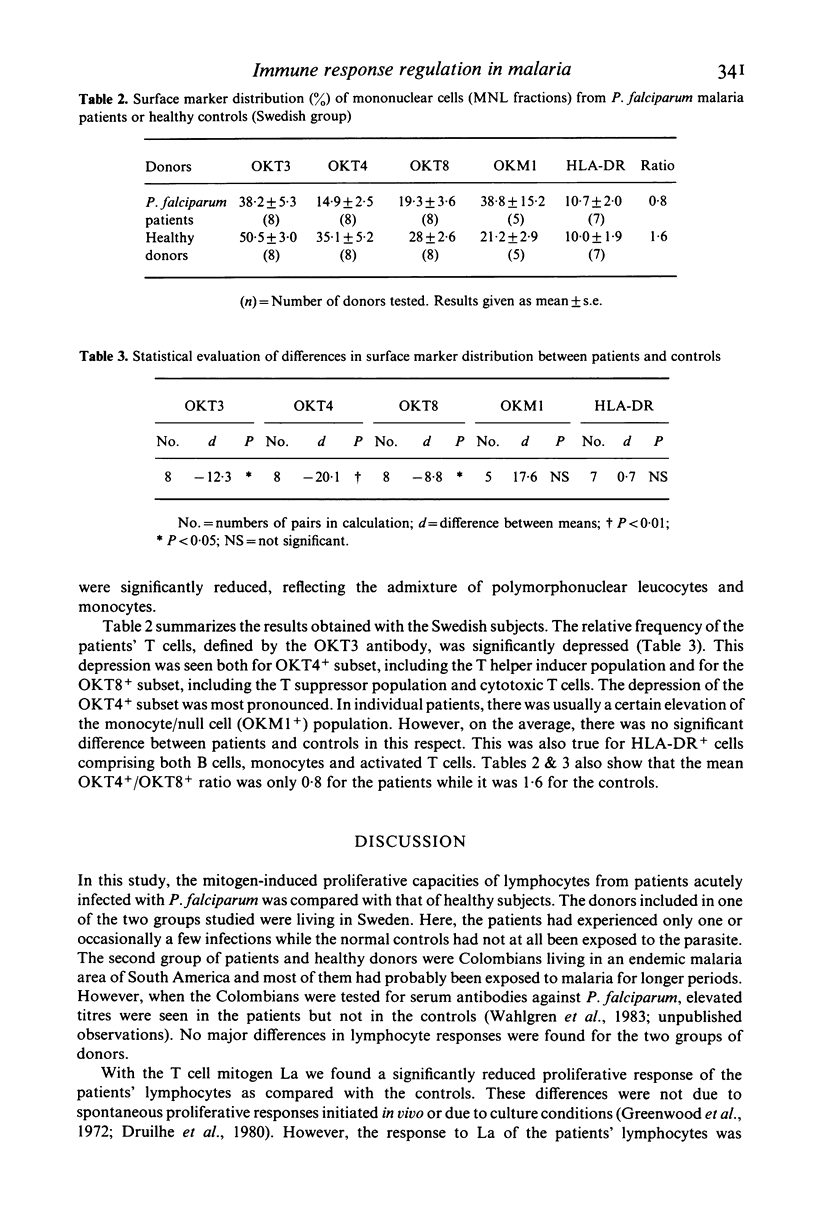
The mitogen-induced DNA synthesis in vitro in lymphocytes from 20 patients acutely ill with Plasmodium falciparum malaria was compared with that of 16 healthy donors. Within both groups part of the donors were individuals who had only experienced short exposure or none at all to the parasite (Sweden) while the other part were donors living in a malaria endemic area (Colombia). The proliferative response to the T cell mitogen La (leucoagglutinin from PHA) of the patients was significantly reduced as compared with that of the controls. With pokeweed mitogen which stimulates T cells and induces a T cell-dependent activation of B cells, no difference between patients or controls was seen. The results were similar for the donors of different geographical origin and malaria background. Lymphocytes and monocytes from the peripheral blood of these donors were also studied for surface marker distribution by means of monoclonal antibodies. Both the absolute and the relative frequencies of T cells in the blood of the malaria patients were significantly reduced as compared with the controls. Furthermore, in almost all eight patients tested, the ratio between T4+ T cells (including the helper/inducer subsets) and T8+ T cells (including the suppressor and cytotoxic subsets) were below 1:1 while they were close to 2:1 in the controls. The results indicate that the relative frequency of T8+ T cells, expressed as percentage total T cells (T3+) was significantly elevated in the P. falciparum patients. The possible relationship between this imbalance and the irregular La response of the patients lymphocytes requires further investigation of lymphocyte function.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Corrêa M., Narayanan P. R., Miller H. C. Suppressive activity of splenic adherent cells from Plasmodium chabaudi-infected mice. J Immunol. 1980 Aug;125(2):749–754. [PubMed] [Google Scholar]
- Engleman E. G., Benike C., Osborne B., Goldsby R. Functional characteristics of human T-cell subpopulations distinguished by a monoclonal antibody. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1607–1611. doi: 10.1073/pnas.77.3.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks D., Bradley B. A. Statistics in cellular typing. Tissue Antigens. 1977 Jul;10(1):1–15. doi: 10.1111/j.1399-0039.1977.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Gabrielsen A. A., Jr, Jensen J. B. Mitogenic activity of extracts from continuous cultures of Plasmodium falciparum. Am J Trop Med Hyg. 1982 May;31(3 Pt 1):441–448. doi: 10.4269/ajtmh.1982.31.441. [DOI] [PubMed] [Google Scholar]
- Golenser J., Shmuel Z., Spira D. T. Aspects of immunosuppression during Plasmodium berghei infection in rats. Trop Geogr Med. 1981 Dec;33(4):347–354. [PubMed] [Google Scholar]
- Greenwood B. M., Bradley-Moore A. M., Bryceson A. D., Palit A. Immunosuppression in children with malaria. Lancet. 1972 Jan 22;1(7743):169–172. doi: 10.1016/s0140-6736(72)90569-7. [DOI] [PubMed] [Google Scholar]
- HURVITZ D., HIRSCHHORN K. SUPPRESSION OF IN VITRO LYMPHOCYTE RESPONSES BY CHLOROQUINE. N Engl J Med. 1965 Jul 1;273:23–26. doi: 10.1056/NEJM196507012730105. [DOI] [PubMed] [Google Scholar]
- Haynes B. F. Human T lymphocyte antigens as defined by monoclonal antibodies. Immunol Rev. 1981;57:127–161. doi: 10.1111/j.1600-065x.1981.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Leuchars E., Davies A. J. The immunological response of CBA mice to P. yoelii. II. The passive transfer of immunity with serum and cells. Immunology. 1978 Jan;34(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Moore D. L., Heyworth B., Brown J. PHA-induced lymphocyte transformations in leucocyte cultures from malarious, malnourished and control Gambian children. Clin Exp Immunol. 1974 Aug;17(4):647–656. [PMC free article] [PubMed] [Google Scholar]
- Norby S. W., Alger N. E. Plasmodium berghei: the in vitro immune response. Exp Parasitol. 1981 Feb;51(1):104–115. doi: 10.1016/0014-4894(81)90047-3. [DOI] [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- SPIEGELBERG H. L., WEIGLE W. O. THE CATABOLISM OF HOMOLOGOUS AND HETEROLOGOUS 7S GAMMA GLOBULIN FRAGMENTS. J Exp Med. 1965 Mar 1;121:323–338. doi: 10.1084/jem.121.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira D. T., Golenser J., Gery I. The reactivity of spleen cells from malarious rats to non-specific mitogens. Clin Exp Immunol. 1976 Apr;24(1):139–145. [PMC free article] [PubMed] [Google Scholar]
- Strambachovà-McBride J., Micklem H. S. Immunosuppression in murine malaria. III. Induction of tolerance and of immunological memory by soluble bovine serum albumin. Immunology. 1979 Mar;36(3):607–614. [PMC free article] [PubMed] [Google Scholar]
- Strickland G. T., DeSilva S., Sayles P. C. Lymphocyte changes in murine and human malaria. Tropenmed Parasitol. 1979 Mar;30(1):35–42. [PubMed] [Google Scholar]
- Taylor D. W., Crum S. R., Kramer K. J., Siddiqui W. A. Alterations in the distribution and proliferative responses of rhesus monkey peripheral blood and spleen cells during malaria (Plasmodium knowlesi) infection. Infect Immun. 1980 May;28(2):502–507. doi: 10.1128/iai.28.2.502-507.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. W., Siddiqui W. A. Effect of falciparum malaria infection on the in vitro mitogen responses of spleen and peripheral blood lymphocytes from owl monkeys. Am J Trop Med Hyg. 1978 Jul;27(4):738–742. doi: 10.4269/ajtmh.1978.27.738. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A. Inhibition of mitogen-induced lymphocyte proliferative responses by quinine. Am J Trop Med Hyg. 1978 Mar;27(2 Pt 1):354–356. doi: 10.4269/ajtmh.1978.27.354. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A., Rowan-Kelly B., O'Keefe D. E. Effect of mefloquine on the immune response in mice. Trans R Soc Trop Med Hyg. 1979;73(4):388–390. doi: 10.1016/0035-9203(79)90160-3. [DOI] [PubMed] [Google Scholar]
- Thong Y. H., Ferrante A., Rowan-Kelly B. Primaquine inhibits mitogen-induced human lymphocyte proliferative responses. Trans R Soc Trop Med Hyg. 1978;72(5):537–539. doi: 10.1016/0035-9203(78)90181-5. [DOI] [PubMed] [Google Scholar]
- Thurman G. B., Strong D. M., Ahmed A., Green S. S., Sell K. W., Hartzman R. J., Bach F. H. Human mixed lymphocyte cultures. Evaluation of a microculture technique utilizing the Multiple Automated Sample Harvester (MASH). Clin Exp Immunol. 1973 Oct;15(2):289–302. [PMC free article] [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. An in vitro assay for T cell immunity to malaria in mice. J Immunol. 1976 May;116(5):1280–1283. [PubMed] [Google Scholar]
- Weinbaum F. I., Evans C. B., Tigelaar R. E. Immunity to Plasmodium Berghei yoelii in mice. I. The course of infection in T cell and B cell deficient mice. J Immunol. 1976 Nov;117(5 PT2):1999–2005. [PubMed] [Google Scholar]
- Weinbaum F. I., Weintraub J., Nkrumah F. K., Evans C. B., Tigelaar R. E., Rosenberg Y. J. Immunity to Plasmodium berghei yoelii in mice. II. Specific and nonspecific cellular and humoral responses during the course of infection. J Immunol. 1978 Aug;121(2):629–636. [PubMed] [Google Scholar]
- Wells R. A., Pavanand K., Zolyomi S., Permpanich B., Macdermott R. P. Anti-lymphocytotoxic antibodies in sera of Thai adults infected with Plasmodium falciparum or Plasmodium vivax. Clin Exp Immunol. 1980 Mar;39(3):663–667. [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Brown J. Malaria antigen-specific T-cell responsiveness during infection with Plasmodium falciparum. Clin Exp Immunol. 1977 Sep;29(3):401–407. [PMC free article] [PubMed] [Google Scholar]
- Wyler D. J., Oppenheim J. J. Lymphocyte transformation in human Plasmodium falciparum malaria. J Immunol. 1974 Aug;113(2):449–454. [PubMed] [Google Scholar]
- Wyler D. J. Peripheral lymphocyte subpopulations in human falciparum malaria. Clin Exp Immunol. 1976 Mar;23(3):471–476. [PMC free article] [PubMed] [Google Scholar]
- Zarling J. M., Kung P. C. Monoclonal antibodies which distinguish between human NK cells and cytotoxic T lymphocytes. Nature. 1980 Nov 27;288(5789):394–396. doi: 10.1038/288394a0. [DOI] [PubMed] [Google Scholar]


