Abstract
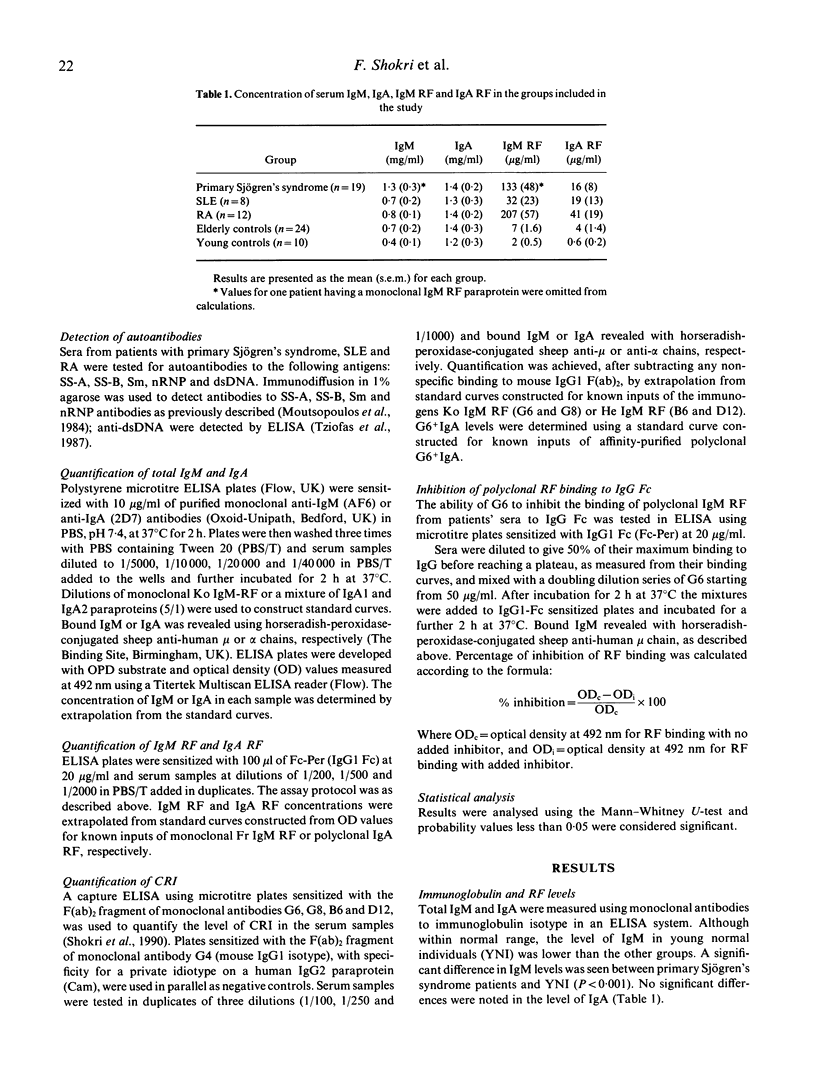
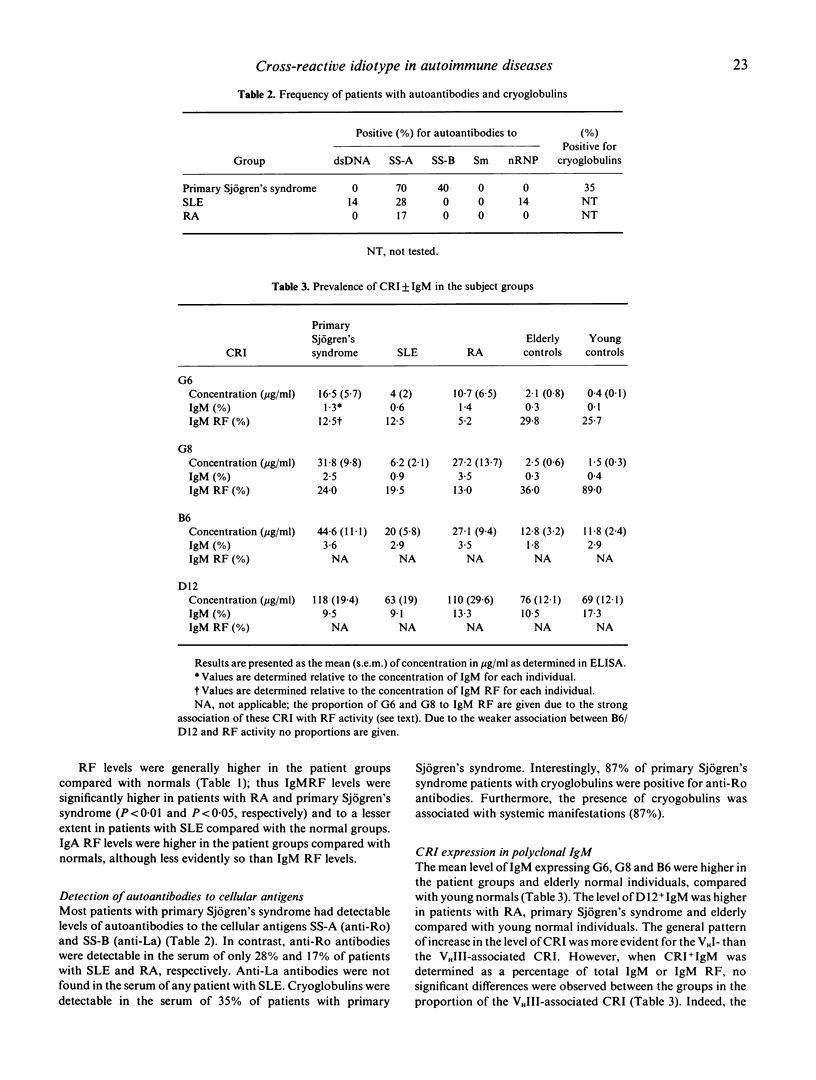
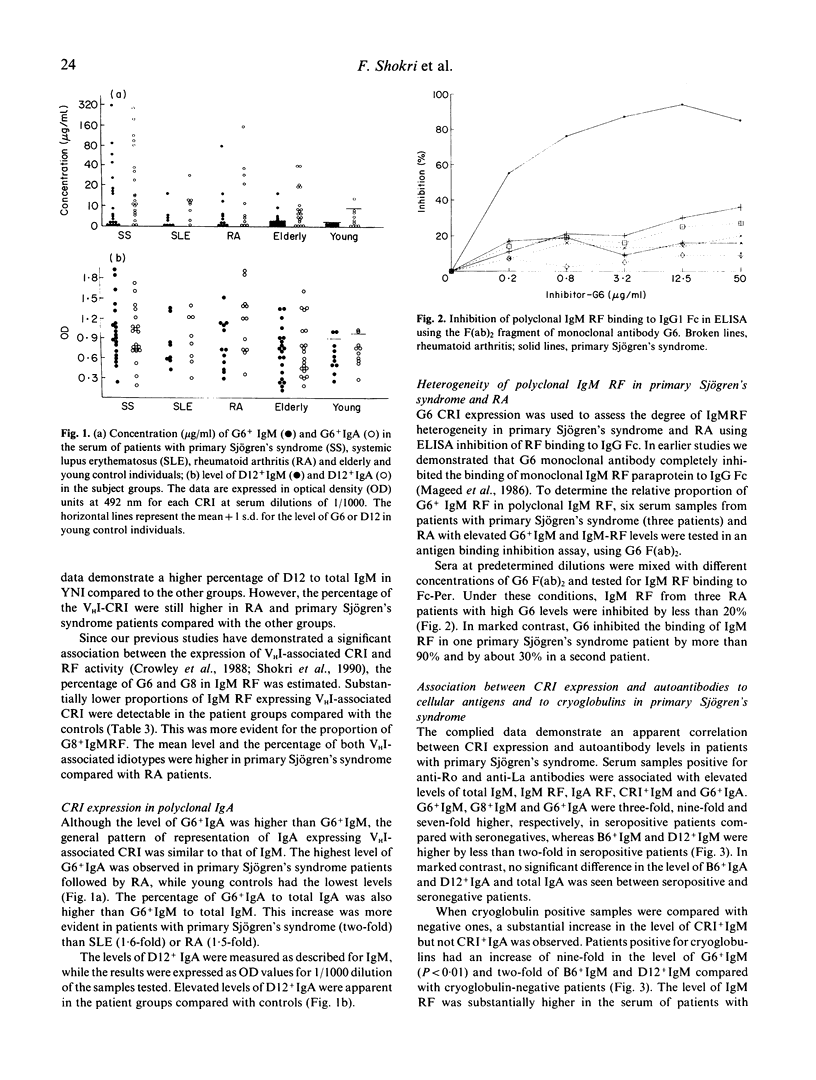
The aetiology of sustained autoantibody production in human autoimmune diseases is unknown. Evidence for structural similarities and common clonal origin among autoantibodies have been demonstrated through the expression of cross-reactive idiotype (CRI). In the present study we use four monoclonal antibodies (MoAbs) with specificity for non-overlapping CRI on human rheumatoid factor (RF) autoantibodies to define the structural features of polyclonal RF characteristic of patients with autoimmune rheumatic diseases. The pattern of CRI expression in the serum of 12 patients with rheumatoid arthritis (RA), eight with systemic lupus erythematosus (SLE) and 20 with primary Sjögren's syndrome and 34 normal individuals were determined in parallel with the level of IgM RF, IgA RF and autoantibodies to the cellular antigens SS-A, SS-B, Sm, nRNP and dsDNA and cryoglobulins. The results demonstrate significant elevation in the level of IgM and IgA expressing VHI (G6 and G8) and VHIII (B6 and D12) associated CRI in the serum of patients with autoimmune rheumatic diseases compared with normal individuals. These increases paralleled, but did not equal the increase in the level of immunoglobulins and RF. However, when expressed as proportion of immunoglobulin, only the VHI-associated CRI were significantly elevated in patients compared with normal individuals. The proportion of IgM RF expressing the VHI-associated CRI was higher in patients with Sjögren's syndrome compared with SLE and RA. Furthermore, the proportion of IgA RF expressing the G6 CRI was higher than G6+ IgM RF. These findings imply that different mechanisms contribute to RF production in autoimmune diseases. It is suggested that polyconal B cell activation is likely to be a contributing mechanism. However, such polyclonal activation is unlikely to be random since a selective elevation in the level of specific autoantibodies and VHI-associated CRI is observed. Furthermore, the data demonstrate that a proportion of autoantibodies in autoimmune diseases are immunoglobulin germline gene encoded. This is more evident in some patients with primary Sjögren's syndrome, where RF is likely to be oligoclonal or monoclonal in individuals with lymphoproliferation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albutt E. C., Hawker P. C., Hine K. R., Northam B. E. Diagnosis of gamma heavy-chain disease. Ann Clin Biochem. 1981 Jul;18(Pt 4):207–210. doi: 10.1177/000456328101800403. [DOI] [PubMed] [Google Scholar]
- Alexander E. L., Hirsch T. J., Arnett F. C., Provost T. T., Stevens M. B. Ro(SSA) and La(SSB) antibodies in the clinical spectrum of Sjögren's syndrome. J Rheumatol. 1982 Mar-Apr;9(2):239–246. [PubMed] [Google Scholar]
- BUNIM J. J., BUCHANAN W. W., WERTLAKE P. T., SOKOLOFF L., BLOCH K. J., BECK J. S., ALEPA F. P. CLINICAL, PATHOLOGIC, AND SEROLOGIC STUDIES IN SJOEGREN'S SYNDROME; COMBINED CLINICAL STAFF CONFERENCE AT THE NATIONAL INSTITUTES OF HEALTH. Ann Intern Med. 1964 Sep;61:509–530. doi: 10.7326/0003-4819-61-3-509. [DOI] [PubMed] [Google Scholar]
- Bresnihan B., Jasin H. E. Suppressor function of peripheral blood mononuclear cells in normal individuals and in patients with systemic lupus erythematosus. J Clin Invest. 1977 Jan;59(1):106–116. doi: 10.1172/JCI108607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley J. J., Goldfien R. D., Schrohenloher R. E., Spiegelberg H. L., Silverman G. J., Mageed R. A., Jefferis R., Koopman W. J., Carson D. A., Fong S. Incidence of three cross-reactive idiotypes on human rheumatoid factor paraproteins. J Immunol. 1988 May 15;140(10):3411–3418. [PubMed] [Google Scholar]
- Crowley J. J., Mageed R. A., Silverman G. J., Chen P. P., Kozin F., Erger R. A., Jefferis R., Carson D. A. The incidence of a new human cross-reactive idiotype linked to subgroup VHIII heavy chains. Mol Immunol. 1990 Jan;27(1):87–94. doi: 10.1016/0161-5890(90)90063-6. [DOI] [PubMed] [Google Scholar]
- Davidson A., Manheimer-Lory A., Aranow C., Peterson R., Hannigan N., Diamond B. Molecular characterization of a somatically mutated anti-DNA antibody bearing two systemic lupus erythematosus-related idiotypes. J Clin Invest. 1990 May;85(5):1401–1409. doi: 10.1172/JCI114584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersimonian H., Schwartz R. S., Barrett K. J., Stollar B. D. Relationship of human variable region heavy chain germ-line genes to genes encoding anti-DNA autoantibodies. J Immunol. 1987 Oct 1;139(7):2496–2501. [PubMed] [Google Scholar]
- Fong S., Chen P. P., Gilbertson T. A., Weber J. R., Fox R. I., Carson D. A. Expression of three cross-reactive idiotypes on rheumatoid factor autoantibodies from patients with autoimmune diseases and seropositive adults. J Immunol. 1986 Jul 1;137(1):122–128. [PubMed] [Google Scholar]
- Fox R. I., Chen P., Carson D. A., Fong S. Expression of a cross-reactive idiotype on rheumatoid factor in patients with Sjogren's syndrome. J Immunol. 1986 Jan;136(2):477–483. [PubMed] [Google Scholar]
- Gharavi A. E., Chu J. L., Elkon K. B. Autoantibodies to intracellular proteins in human systemic lupus erythematosus are not due to random polyclonal B cell activation. Arthritis Rheum. 1988 Nov;31(11):1337–1345. doi: 10.1002/art.1780311101. [DOI] [PubMed] [Google Scholar]
- Goldfien R. D., Chen P. J., Kipps T. J., Starkebaum G., Heitzmann J. G., Radoux V., Fong S., Carson D. A. Genetic analysis of human B cell hybridomas expressing a cross-reactive idiotype. J Immunol. 1987 Feb 1;138(3):940–944. [PubMed] [Google Scholar]
- Hansen B., Manthorpe R. Antibodies against SS-B/La and SS-A/Ro antigens in patients with primary Sjögren's syndrome. Scand J Rheumatol Suppl. 1986;61:93–97. [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Isenberg D., Dudeney C., Wojnaruska F., Bhogal B. S., Rauch J., Schattner A., Naparstek Y., Duggan D. Detection of cross reactive anti-DNA antibody idiotypes on tissue-bound immunoglobulins from skin biopsies of lupus patients. J Immunol. 1985 Jul;135(1):261–264. [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Jaafar M. I., Lowe J. A., Ling N. R., Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins--V. Reactivity of a panel of monoclonal antibodies with sub-fragments of human Fc gamma and abnormal paraproteins having deletions. Mol Immunol. 1983 Jun;20(6):679–686. doi: 10.1016/0161-5890(83)90012-3. [DOI] [PubMed] [Google Scholar]
- Kassan S. S., Thomas T. L., Moutsopoulos H. M., Hoover R., Kimberly R. P., Budman D. R., Costa J., Decker J. L., Chused T. M. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978 Dec;89(6):888–892. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- Kipps T. J., Tomhave E., Pratt L. F., Duffy S., Chen P. P., Carson D. A. Developmentally restricted immunoglobulin heavy chain variable region gene expressed at high frequency in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5913–5917. doi: 10.1073/pnas.86.15.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel H. G., Agnello V., Joslin F. G., Winchester R. J., Capra J. D. Cross-idiotypic specificity among monoclonal IgM proteins with anti- -globulin activity. J Exp Med. 1973 Feb 1;137(2):331–342. doi: 10.1084/jem.137.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel H. G., Winchester R. J., Joslin F. G., Capra J. D. Similarities in the light chains of anti-gamma-globulins showing cross-idiotypic specificities. J Exp Med. 1974 Jan 1;139(1):128–136. doi: 10.1084/jem.139.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoyi E., Nisonoff A. Preparation of F(ab')2 fragments from mouse IgG of various subclasses. J Immunol Methods. 1983 Jan 28;56(2):235–243. doi: 10.1016/0022-1759(83)90415-5. [DOI] [PubMed] [Google Scholar]
- Mageed R. A., Goodall D. M., Jefferis R. A highly conserved conformational idiotope on human IgM rheumatoid factor paraproteins of the Wa cross-reactive idiotype family defined by a monoclonal antibody. Rheumatol Int. 1990;10(2):57–63. doi: 10.1007/BF02274784. [DOI] [PubMed] [Google Scholar]
- Miyasaka N., Yamaoka K., Sato K., Kubota T., Okuda M., Nishioka K., Ohya Y., Yamamoto K. An analysis of polyclonal B cell activation in Sjögren's syndrome. Characterization of B cell lines spontaneously established from the peripheral blood. Scand J Rheumatol Suppl. 1986;61:123–126. [PubMed] [Google Scholar]
- Moutsopoulos H. M., Giotaki H., Maddison P. J., Mavridis A. C., Drosos A. A., Skopouli F. N. Antibodies to cellular antigens in Greek patients with autoimmune rheumatic diseases: anti-Ro(SSA) antibody a possible marker of penicillamine-D intolerance. Ann Rheum Dis. 1984 Apr;43(2):285–287. doi: 10.1136/ard.43.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newkirk M. M., Mageed R. A., Jefferis R., Chen P. P., Capra J. D. Complete amino acid sequences of variable regions of two human IgM rheumatoid factors, BOR and KAS of the Wa idiotypic family, reveal restricted use of heavy and light chain variable and joining region gene segments. J Exp Med. 1987 Aug 1;166(2):550–564. doi: 10.1084/jem.166.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos N. M., Kintzios J. A. Differentiation of pathological conditions by visual evaluation of serum protein electrophoretic patterns. Proc Soc Exp Biol Med. 1967 Jul;125(3):927–930. doi: 10.3181/00379727-125-32242. [DOI] [PubMed] [Google Scholar]
- Pascali E., Pezzoli A., Chiarandini A. Immunofixation: application to the identification of "difficult" monoclonal components. Clin Chem. 1982 Jun;28(6):1404–1405. [PubMed] [Google Scholar]
- Pollard K. M., Steele R., Hogg S., Webb J. Measurement of serum DNA binding in chronic active hepatitis and systemic lupus erythematosus using the Farr assay. Rheumatol Int. 1986;6(3):139–144. doi: 10.1007/BF00270351. [DOI] [PubMed] [Google Scholar]
- Preud'homme J. L., Seligmann M. Anti-human immunoglobulin G activity of membrane-bound monoclonal immunoglobulin M in lymphoproliferative disorders. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2132–2135. doi: 10.1073/pnas.69.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Sanz I., Dang H., Takei M., Talal N., Capra J. D. VH sequence of a human anti-Sm autoantibody. Evidence that autoantibodies can be unmutated copies of germline genes. J Immunol. 1989 Feb 1;142(3):883–887. [PubMed] [Google Scholar]
- Schiff C., Milili M., Hue I., Rudikoff S., Fougereau M. Genetic basis for expression of the idiotypic network. One unique Ig VH germline gene accounts for the major family of Ab1 and Ab3 (Ab1') antibodies of the GAT system. J Exp Med. 1986 Mar 1;163(3):573–587. doi: 10.1084/jem.163.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- Shoenfeld Y., Isenberg D. A., Rauch J., Madaio M. P., Stollar B. D., Schwartz R. S. Idiotypic cross-reactions of monoclonal human lupus autoantibodies. J Exp Med. 1983 Sep 1;158(3):718–730. doi: 10.1084/jem.158.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri F., Mageed R. A., Tunn E., Bacon P. A., Jefferis R. Qualitative and quantitative expression of VHI associated cross reactive idiotopes within IgM rheumatoid factor from patients with early synovitis. Ann Rheum Dis. 1990 Mar;49(3):150–154. doi: 10.1136/ard.49.3.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon G., Schiffenbauer J., Keiser H. D., Diamond B. Use of monoclonal antibodies to identify shared idiotypes on human antibodies to native DNA from patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1983 Feb;80(3):850–854. doi: 10.1073/pnas.80.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai S., Shimizu S., Konda S. Lymphoproliferative disorders in Japanese patients with Sjögren's syndrome. Scand J Rheumatol Suppl. 1986;61:118–122. [PubMed] [Google Scholar]
- Sugai S., Shimizu S., Tachibana J., Imaoka S., Konda S. A high incidence of rheumatoid factor idiotypes in monoclonal proteins in the serum and in lymphoma cells in patients with Sjögren's syndrome. J Autoimmun. 1989 Aug;2(4):471–476. doi: 10.1016/0896-8411(89)90177-7. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Tzioufas A. G., Manoussakis M. N., Drosos A. A., Silis G., Gharavi A. E., Moutsopoulos H. M. Enzyme immunoassays for the detection of IgG and IgM anti-dsDNA antibodies: clinical significance and specificity. Clin Exp Rheumatol. 1987 Jul-Sep;5(3):247–253. [PubMed] [Google Scholar]
- Walters M. T., Stevenson F. K., Herbert A., Cawley M. I., Smith J. L. Lymphoma in Sjögren's syndrome: urinary monoclonal free light chains as a diagnostic aid and a means of tumour monitoring. Scand J Rheumatol Suppl. 1986;61:114–117. [PubMed] [Google Scholar]


