Abstract
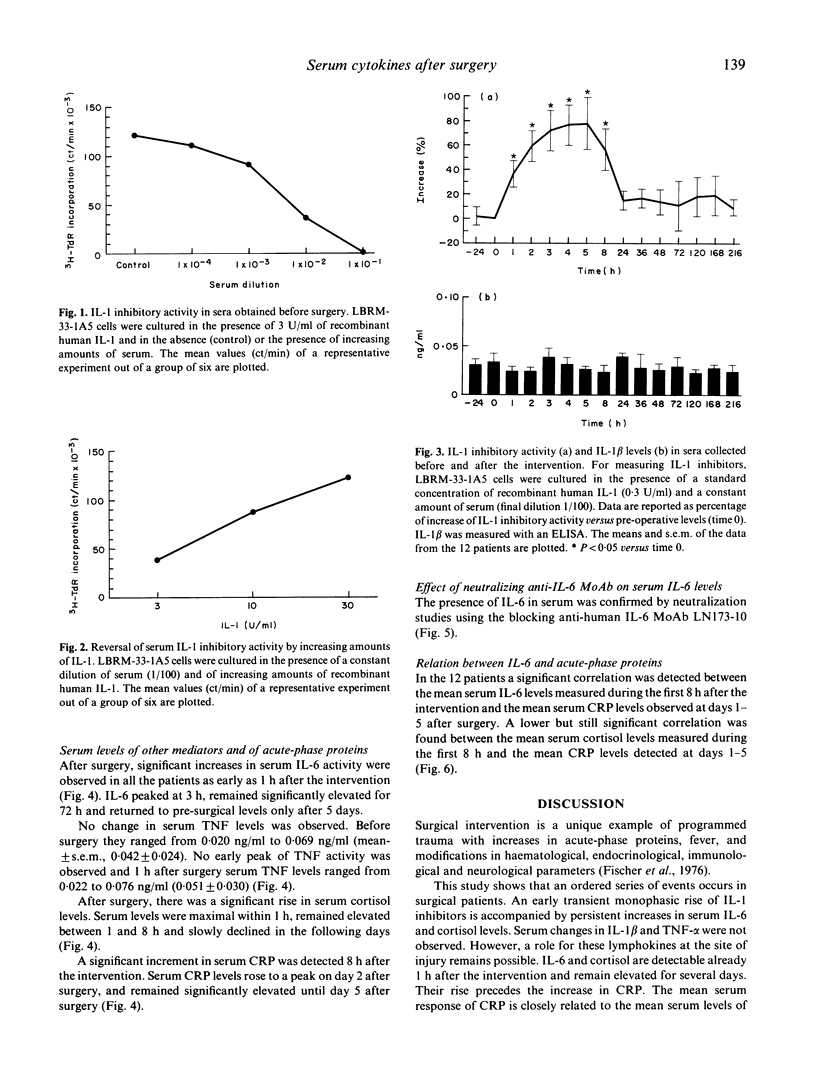
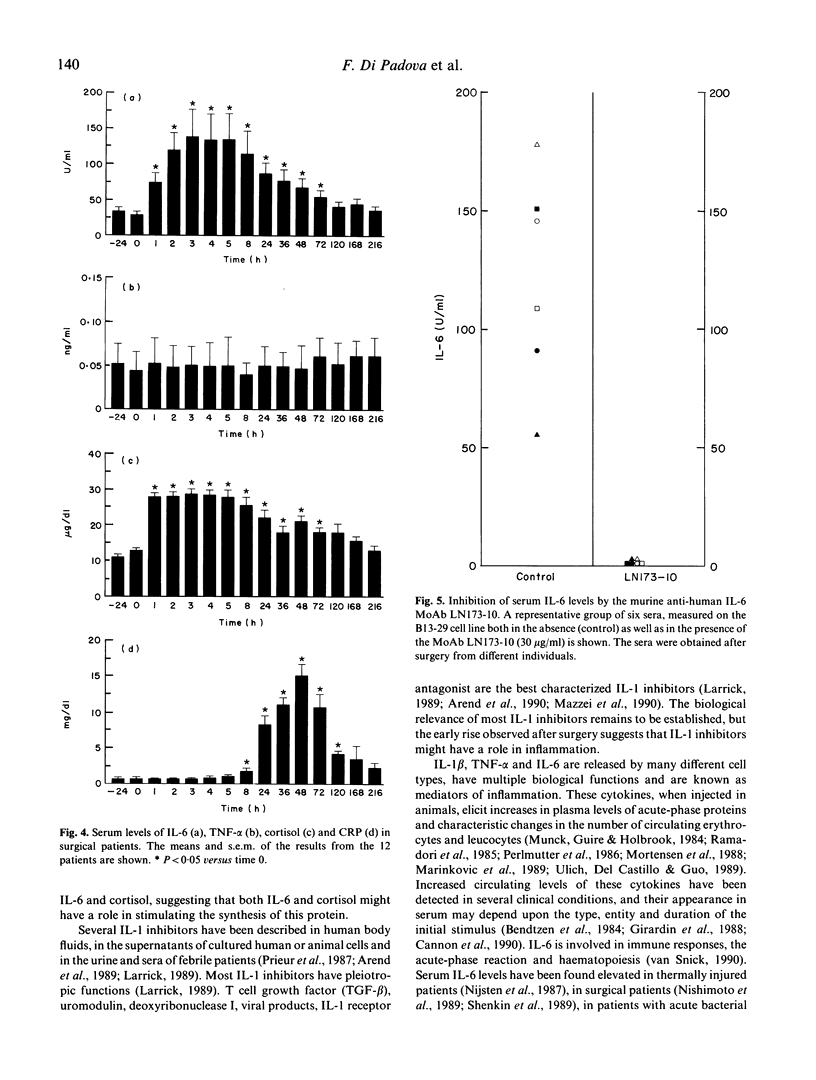
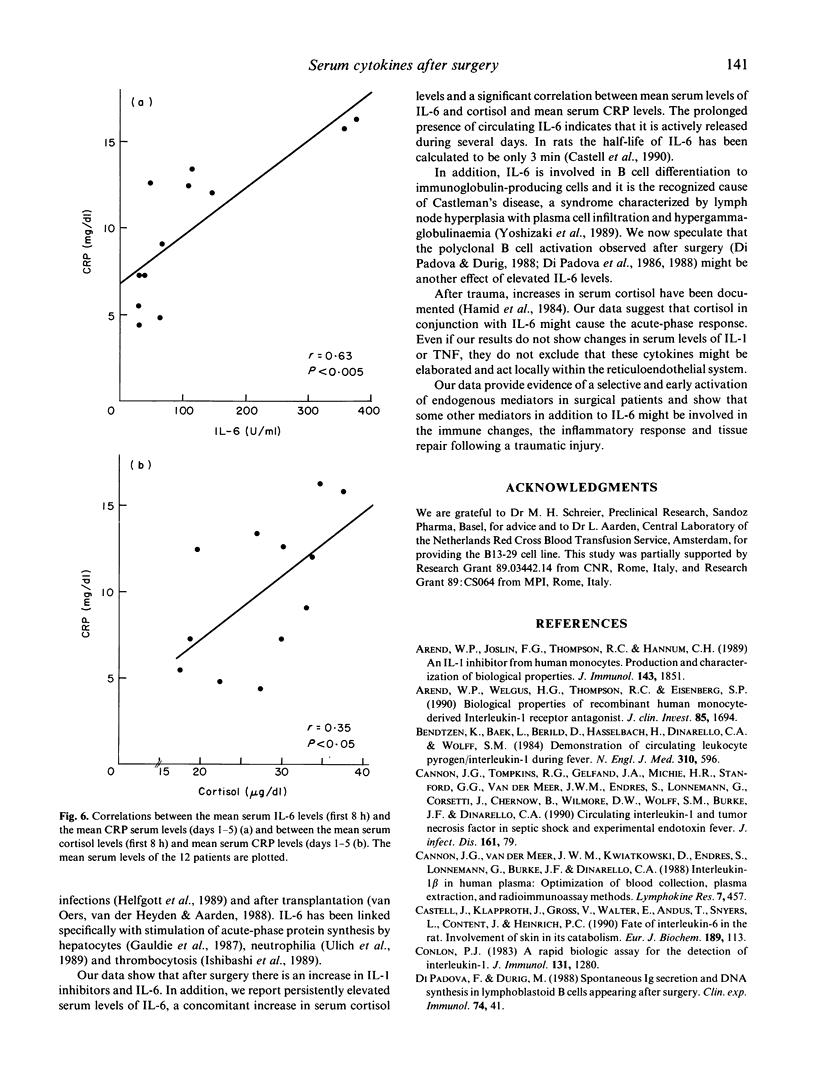
After trauma, inflammatory, immunological and hormonal changes are well documented. Surgical intervention is a form of programmed trauma. Through the study of surgical patients, changes in early endogenous mediators of inflammation, immune response and tissue repair can be investigated. Here we analysed changes in serum levels of IL-1 inhibitors, IL-1 beta, IL-6, tumour necrosis factor-alpha (TNF-alpha) and cortisol in patients undergoing elective surgery. C-reactive protein (CRP) was measured as a marker of the acute-phase response. Rises in serum levels of IL-1 inhibitors, IL-6 and cortisol were detected as early as 1 h after the intervention. Peak levels were reached between 2 and 5 h. Serum levels of IL-6 and cortisol remained elevated for several days implying a persistent production. Serum levels of IL-1 and TNF did not change after the intervention. CRP levels peaked on day 2. The communication system sustained by endogenous mediators is activated after surgery as shown by selective changes in IL-1 inhibitors, IL-6 and cortisol. These mediators have different kinetics in serum and IL-6 is not the only early mediator detected. Some IL-1 inhibitors might be involved in the immunological depression observed after major surgery, in the regulation of the inflammatory response or in tissue repair. IL-6 and cortisol seem to act synergistically to activate the acute-phase response. A systemic role for IL-1 and TNF is not evident, even if the possibility that these lymphokines may act locally is not ruled out.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Joslin F. G., Thompson R. C., Hannum C. H. An IL-1 inhibitor from human monocytes. Production and characterization of biologic properties. J Immunol. 1989 Sep 15;143(6):1851–1858. [PubMed] [Google Scholar]
- Arend W. P., Welgus H. G., Thompson R. C., Eisenberg S. P. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest. 1990 May;85(5):1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtzen K., Baek L., Berild D., Hasselbach H., Dinarello C. A., Wolff S. M. Demonstration of circulating leukocytic pyrogen/interleukin-1 during fever. N Engl J Med. 1984 Mar 1;310(9):596–596. doi: 10.1056/NEJM198403013100915. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Tompkins R. G., Gelfand J. A., Michie H. R., Stanford G. G., van der Meer J. W., Endres S., Lonnemann G., Corsetti J., Chernow B. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990 Jan;161(1):79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., van der Meer J. W., Kwiatkowski D., Endres S., Lonnemann G., Burke J. F., Dinarello C. A. Interleukin-1 beta in human plasma: optimization of blood collection, plasma extraction, and radioimmunoassay methods. Lymphokine Res. 1988 Winter;7(4):457–467. [PubMed] [Google Scholar]
- Castell J., Klapproth J., Gross V., Walter E., Andus T., Snyers L., Content J., Heinrich P. C. Fate of interleukin-6 in the rat. Involvement of skin in its catabolism. Eur J Biochem. 1990 Apr 20;189(1):113–118. doi: 10.1111/j.1432-1033.1990.tb15466.x. [DOI] [PubMed] [Google Scholar]
- Conlon P. J. A rapid biologic assay for the detection of interleukin 1. J Immunol. 1983 Sep;131(3):1280–1282. [PubMed] [Google Scholar]
- Di Padova F., Di Padova C., Rovagnati P., Tritapepe R. Appearance of spontaneously Ig secreting B cells in human peripheral blood after surgery. Clin Exp Immunol. 1986 Sep;65(3):582–588. [PMC free article] [PubMed] [Google Scholar]
- Di Padova F., Durig M., Di Padova C., Pozzoli M., Tritapepe R. Spontaneous and polyclonal Ig secretion by circulating B cells after surgery. Surgery. 1988 May;103(5):547–552. [PubMed] [Google Scholar]
- Di Padova F., Dürig M. Spontaneous Ig secretion and DNA synthesis in lymphoblastoid B cells appearing after surgery. Clin Exp Immunol. 1988 Oct;74(1):41–46. [PMC free article] [PubMed] [Google Scholar]
- Fischer C. L., Gill C., Forrester M. G., Nakamura R. Quantitation of "acute-phase proteins" postoperatively. Value in detection and monitoring of complications. Am J Clin Pathol. 1976 Nov;66(5):840–846. doi: 10.1093/ajcp/66.5.840. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- Hamid J., Bancewicz J., Brown R., Ward C., Irving M. H., Ford W. L. The significance of changes in blood lymphocyte populations following surgical operations. Clin Exp Immunol. 1984 Apr;56(1):49–57. [PMC free article] [PubMed] [Google Scholar]
- Helfgott D. C., Tatter S. B., Santhanam U., Clarick R. H., Bhardwaj N., May L. T., Sehgal P. B. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989 Feb 1;142(3):948–953. [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Ishibashi T., Kimura H., Shikama Y., Uchida T., Kariyone S., Hirano T., Kishimoto T., Takatsuki F., Akiyama Y. Interleukin-6 is a potent thrombopoietic factor in vivo in mice. Blood. 1989 Sep;74(4):1241–1244. [PubMed] [Google Scholar]
- Larrick J. W. Native interleukin 1 inhibitors. Immunol Today. 1989 Feb;10(2):61–66. doi: 10.1016/0167-5699(89)90308-3. [DOI] [PubMed] [Google Scholar]
- Marinkovic S., Jahreis G. P., Wong G. G., Baumann H. IL-6 modulates the synthesis of a specific set of acute phase plasma proteins in vivo. J Immunol. 1989 Feb 1;142(3):808–812. [PubMed] [Google Scholar]
- Mazzei G. J., Seckinger P. L., Dayer J. M., Shaw A. R. Purification and characterization of a 26-kDa competitive inhibitor of interleukin 1. Eur J Immunol. 1990 Mar;20(3):683–689. doi: 10.1002/eji.1830200332. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Shapiro J., Lin B. F., Douches S., Neta R. Interaction of recombinant IL-1 and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J Immunol. 1988 Apr 1;140(7):2260–2266. [PubMed] [Google Scholar]
- Munck A., Guyre P. M., Holbrook N. J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984 Winter;5(1):25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nijsten M. W., de Groot E. R., ten Duis H. J., Klasen H. J., Hack C. E., Aarden L. A. Serum levels of interleukin-6 and acute phase responses. Lancet. 1987 Oct 17;2(8564):921–921. doi: 10.1016/s0140-6736(87)91413-9. [DOI] [PubMed] [Google Scholar]
- Nishimoto N., Yoshizaki K., Tagoh H., Monden M., Kishimoto S., Hirano T., Kishimoto T. Elevation of serum interleukin 6 prior to acute phase proteins on the inflammation by surgical operation. Clin Immunol Immunopathol. 1989 Mar;50(3):399–401. doi: 10.1016/0090-1229(89)90147-5. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieur A. M., Kaufmann M. T., Griscelli C., Dayer J. M. Specific interleukin-1 inhibitor in serum and urine of children with systemic juvenile chronic arthritis. Lancet. 1987 Nov 28;2(8570):1240–1242. doi: 10.1016/s0140-6736(87)91854-x. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Sipe J. D., Dinarello C. A., Mizel S. B., Colten H. R. Pretranslational modulation of acute phase hepatic protein synthesis by murine recombinant interleukin 1 (IL-1) and purified human IL-1. J Exp Med. 1985 Sep 1;162(3):930–942. doi: 10.1084/jem.162.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkin A., Fraser W. D., Series J., Winstanley F. P., McCartney A. C., Burns H. J., Van Damme J. The serum interleukin 6 response to elective surgery. Lymphokine Res. 1989 Summer;8(2):123–127. [PubMed] [Google Scholar]
- Ulich T. R., del Castillo J., Guo K. Z. In vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCs. Blood. 1989 Jan;73(1):108–110. [PubMed] [Google Scholar]
- Van Oers M. H., Van der Heyden A. A., Aarden L. A. Interleukin 6 (IL-6) in serum and urine of renal transplant recipients. Clin Exp Immunol. 1988 Feb;71(2):314–319. [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K., Matsuda T., Nishimoto N., Kuritani T., Taeho L., Aozasa K., Nakahata T., Kawai H., Tagoh H., Komori T. Pathogenic significance of interleukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989 Sep;74(4):1360–1367. [PubMed] [Google Scholar]


