Abstract
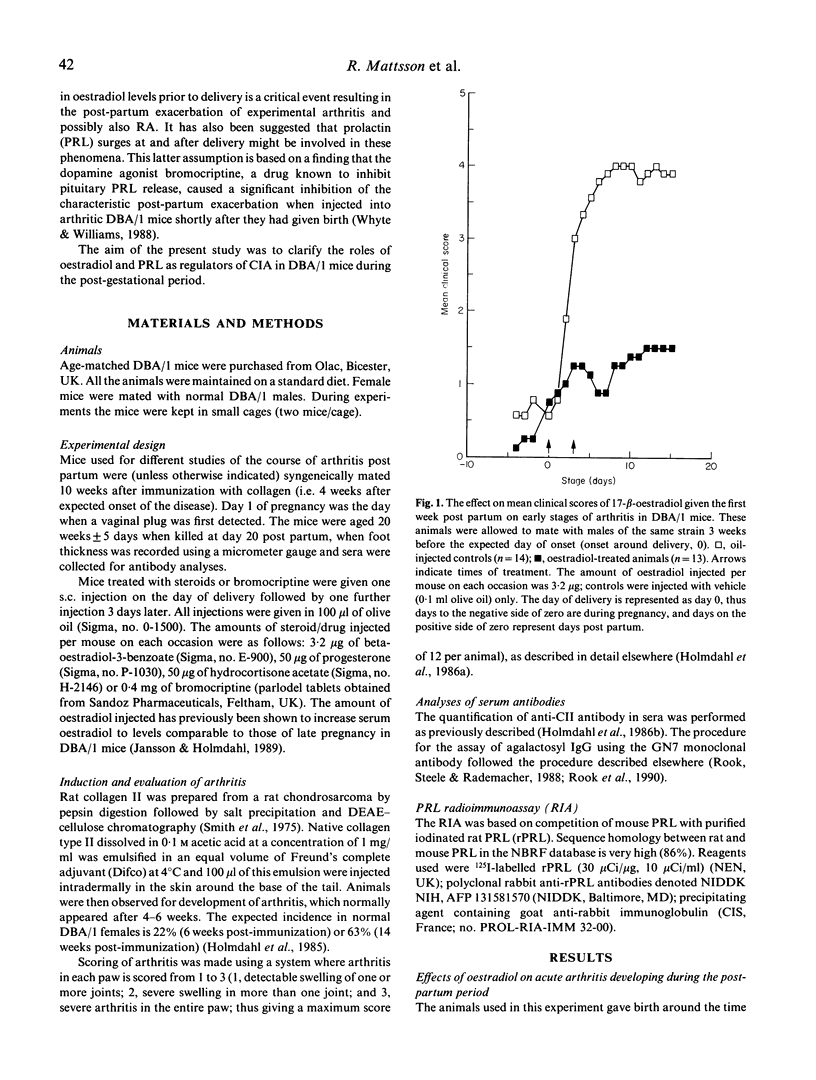
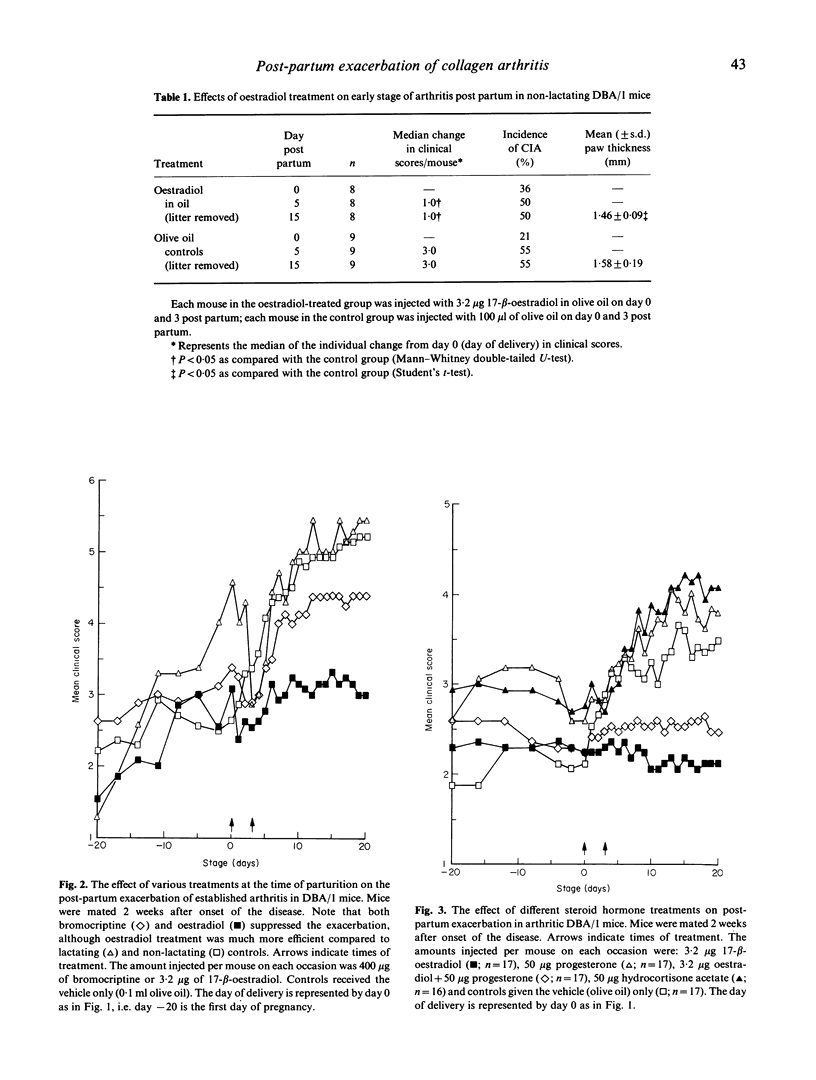
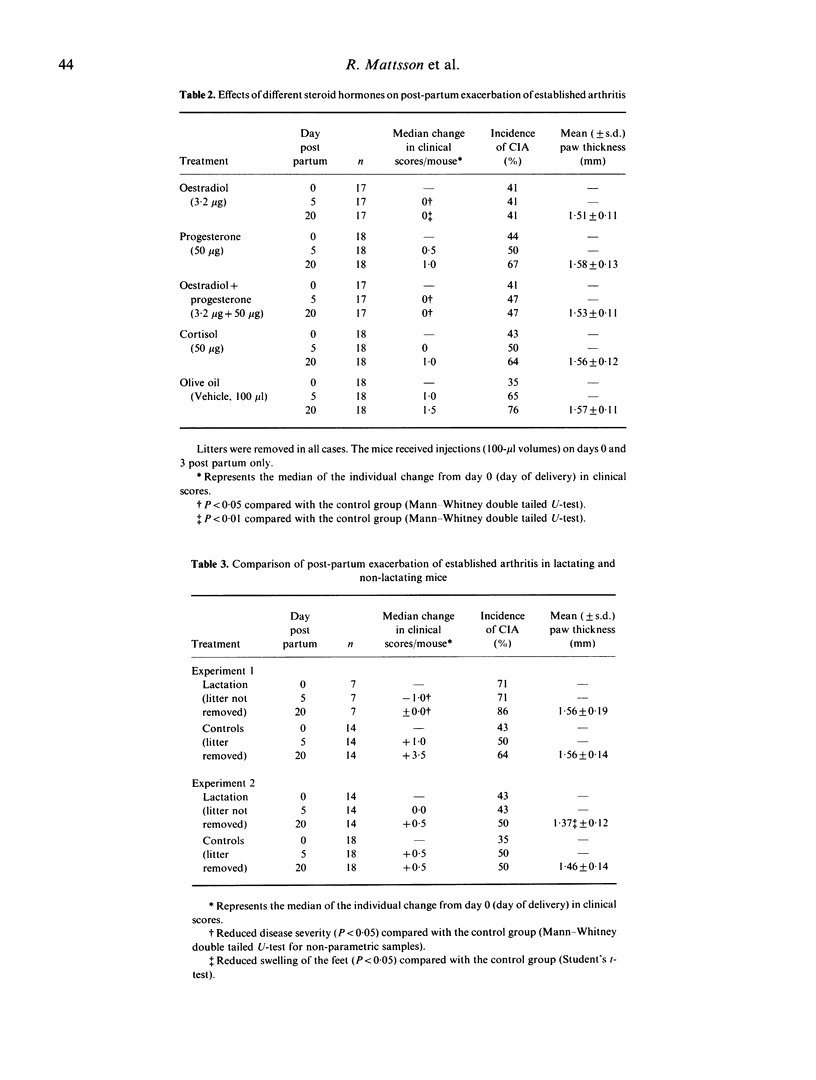
Pregnancy is known to influence the course of rheumatoid arthritis (RA) in women, as well as type II collagen-induced arthritis (CIA) in DBA/1 mice. A characteristic feature is the remission during gestation and the exacerbation of the diseases during the post-partum period. In the case of CIA in DBA 1 mice, two hormonal changes have been assumed to be critical for the induction of the post-partum flare: (i) the fall in steroid hormone levels from those present during pregnancy; and (ii) surges of prolactin (PRL) release at and after delivery. Our results show that treatment with oestradiol during a short period immediately after parturition protects the mouse from a post-partum flare of the disease, and that treatment with bromocriptine, a drug known to inhibit the endogenous PRL release, has a significant though less marked effect. Studies of lactating (i.e. animals with physiological stimulation of endogenous PRL release) and non-lactating arthritic mice revealed no clear-cut differences, indicating that PRL is of minor importance for the induction of the post-partum flare. Some steroids other than oestradiol, which may be implicated in the exacerbation of arthritis, namely progesterone and hydrocortisone, had no clinical effect. Analyses of agalactosyl IgG levels in mice with CIA, and anti-collagen II antibodies in sera collected at the end of the experiments revealed no significant differences between the oestradiol and the control groups. The successful oestradiol treatment of the mice indicates that the drop in endogenous oestradiol levels prior to delivery ends the oestrogen-mediated protection against arthritis during pregnancy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Grossman C. J. Regulation of the immune system by sex steroids. Endocr Rev. 1984 Summer;5(3):435–455. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- Gudelsky G. A., Nansel D. D., Porter J. C. Role of estrogen in the dopaminergic control of prolactin secretion. Endocrinology. 1981 Feb;108(2):440–444. doi: 10.1210/endo-108-2-440. [DOI] [PubMed] [Google Scholar]
- Hazes J. M., Dijkmans B. C., Vandenbroucke J. P., de Vries R. R., Cats A. Reduction of the risk of rheumatoid arthritis among women who take oral contraceptives. Arthritis Rheum. 1990 Feb;33(2):173–179. doi: 10.1002/art.1780330204. [DOI] [PubMed] [Google Scholar]
- Hirahara F., Wooley P. H., Luthra H. S., Coulam C. B., Griffiths M. M., David C. S. Collagen-induced arthritis and pregnancy in mice: the effects of pregnancy on collagen-induced arthritis and the high incidence of infertility in arthritic female mice. Am J Reprod Immunol Microbiol. 1986 Jun;11(2):44–54. doi: 10.1111/j.1600-0897.1986.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Gullberg D., Rubin K., Forsberg P. O., Klareskog L. Incidence of arthritis and autoreactivity of anti-collagen antibodies after immunization of DBA/1 mice with heterologous and autologous collagen II. Clin Exp Immunol. 1985 Dec;62(3):639–646. [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Meyerson B., Klareskog L. Oestrogen induced suppression of collagen arthritis: I. Long term oestradiol treatment of DBA/1 mice reduces severity and incidence of arthritis and decreases the anti type II collagen immune response. Clin Exp Immunol. 1987 Nov;70(2):372–378. [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Andersson M., Hansen C. High antibody response to autologous type II collagen is restricted to H-2q. Immunogenetics. 1986;24(2):84–89. doi: 10.1007/BF00373114. [DOI] [PubMed] [Google Scholar]
- Jansson L., Holmdahl R. Oestrogen induced suppression of collagen arthritis. IV: Progesterone alone does not affect the course of arthritis but enhances the oestrogen-mediated therapeutic effect. J Reprod Immunol. 1989 May;15(2):141–150. doi: 10.1016/0165-0378(89)90033-8. [DOI] [PubMed] [Google Scholar]
- Lahita R. G. Sex steroids and the rheumatic diseases. Arthritis Rheum. 1985 Feb;28(2):121–126. doi: 10.1002/art.1780280202. [DOI] [PubMed] [Google Scholar]
- Neill J. D., Freeman M. E., Tillson S. A. Control of the proestrus surge of prolactin and luteinizing hormone secretion by estrogens in the rat. Endocrinology. 1971 Dec;89(6):1448–1453. doi: 10.1210/endo-89-6-1448. [DOI] [PubMed] [Google Scholar]
- Oka M., Vainio U. Effect of pregnancy on the prognosis and serology of rheumatoid arthritis. Acta Rheumatol Scand. 1966;12(1):47–52. doi: 10.3109/rhe1.1966.12.issue-1-4.06. [DOI] [PubMed] [Google Scholar]
- Okayasu I., Kong Y. M., Rose N. R. Effect of castration and sex hormones on experimental autoimmune thyroiditis. Clin Immunol Immunopathol. 1981 Aug;20(2):240–245. doi: 10.1016/0090-1229(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Ostensen M., Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 1983 Sep;26(9):1155–1159. doi: 10.1002/art.1780260915. [DOI] [PubMed] [Google Scholar]
- Quadri S. K., Oyama T., Spies H. G. Effects of 17 beta-estradiol on serum protlactin levels and on prolactin responses to thyrotropin-releasing hormone in female rhesus monkeys. Endocrinology. 1979 Jun;104(6):1649–1655. doi: 10.1210/endo-104-6-1649. [DOI] [PubMed] [Google Scholar]
- Reduction in incidence of rheumatoid arthritis associated with oral contraceptives. Royal College of General Practitioners' Oral Contraception Study. Lancet. 1978 Mar 18;1(8064):569–571. [PubMed] [Google Scholar]
- Rook G. A., Steele J., Rademacher T. A monoclonal antibody raised by immunising mice with group A streptococci binds to agalactosyl IgG from rheumatoid arthritis. Ann Rheum Dis. 1988 Mar;47(3):247–250. doi: 10.1136/ard.47.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978 Jun 1;147(6):1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutlin E., Haug E., Torjesen P. A. Serum thyrotrophin, prolactin and growth hormone, response to TRH during oestrogen treatment. Acta Endocrinol (Copenh) 1977 Jan;84(1):23–35. doi: 10.1530/acta.0.0840023. [DOI] [PubMed] [Google Scholar]
- Smith B. D., Martin G. R., Miller E. J., Dorfman A., Swarm R. Nature of the collagen synthesized by a transplanted chondrosarcoma. Arch Biochem Biophys. 1975 Jan;166(1):181–186. doi: 10.1016/0003-9861(75)90378-1. [DOI] [PubMed] [Google Scholar]
- Spector T. D., Roman E., Silman A. J. The pill, parity, and rheumatoid arthritis. Arthritis Rheum. 1990 Jun;33(6):782–789. doi: 10.1002/art.1780330604. [DOI] [PubMed] [Google Scholar]
- Strigård K., Holmdahl R., Olsson T. Oestrogen treatment reduces duration of experimental allergic neuritis in rats and suppresses T cell responses to myelin. Acta Neurol Scand. 1990 May;81(5):436–442. doi: 10.1111/j.1600-0404.1990.tb00991.x. [DOI] [PubMed] [Google Scholar]
- Toivanen P., Siikala H., Laiho P., Paavilainen T. Suppression of adjuvant arthritis by estrone in adrenalectomized and ovariectomized rats. Experientia. 1967 Jul 15;23(7):560–561. doi: 10.1007/BF02137970. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke J. P., Witteman J. C., Valkenburg H. A., Boersma J. W., Cats A., Festen J. J., Hartman A. P., Huber-Bruning O., Rasker J. J., Weber J. Noncontraceptive hormones and rheumatoid arthritis in perimenopausal and postmenopausal women. JAMA. 1986 Mar 14;255(10):1299–1303. [PubMed] [Google Scholar]
- Waites G. T., Whyte A. Effect of pregnancy on collagen-induced arthritis in mice. Clin Exp Immunol. 1987 Mar;67(3):467–476. [PMC free article] [PubMed] [Google Scholar]
- Whyte A., Williams R. O. Bromocriptine suppresses postpartum exacerbation of collagen-induced arthritis. Arthritis Rheum. 1988 Jul;31(7):927–928. doi: 10.1002/art.1780310717. [DOI] [PubMed] [Google Scholar]


