Abstract
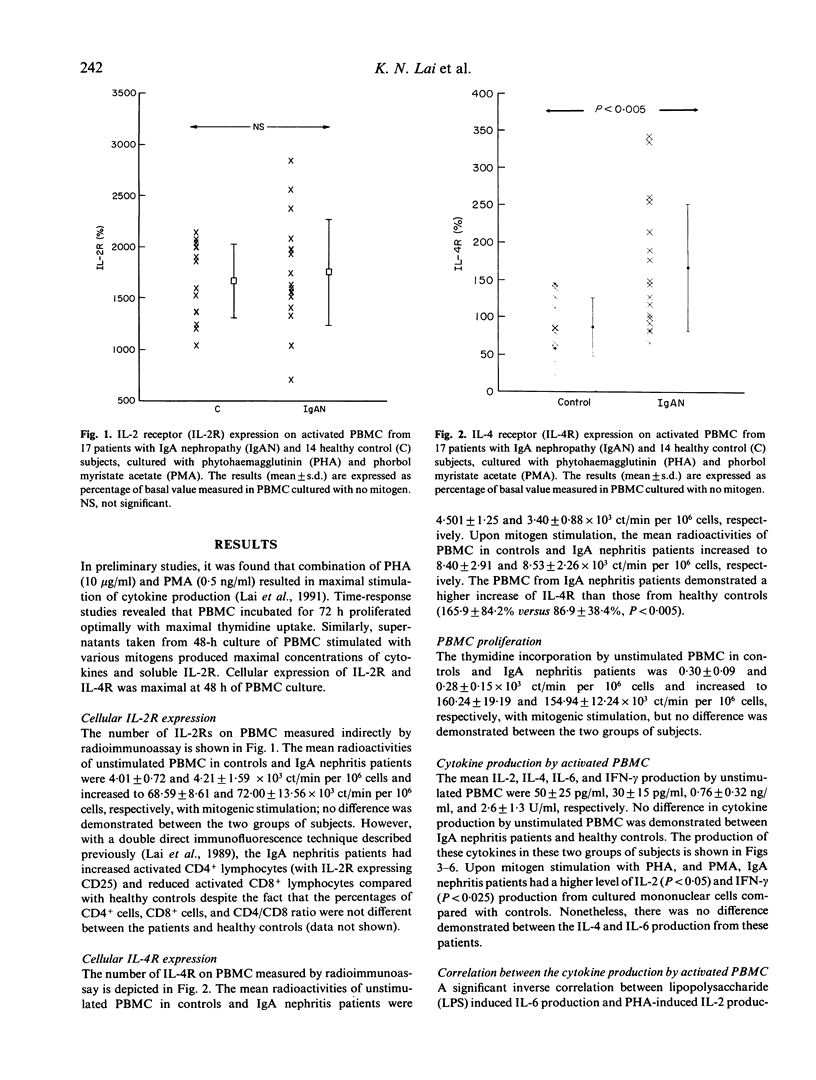
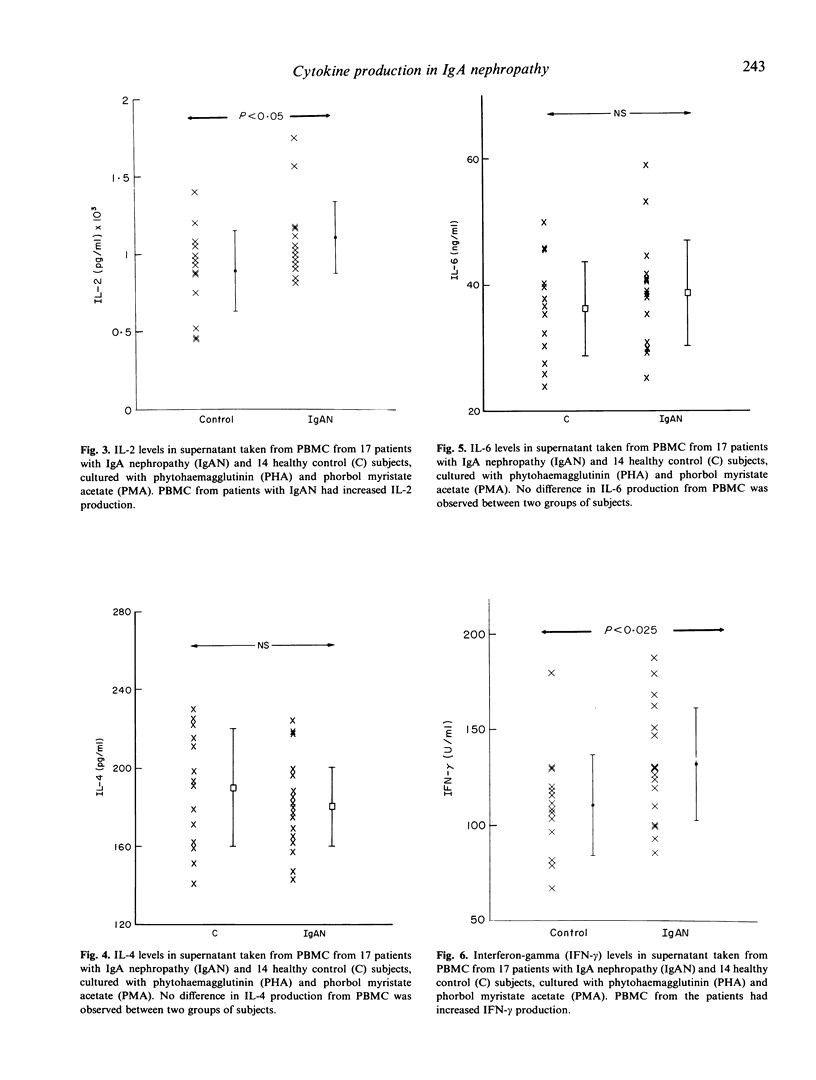
The regulation of cytokine production and T cell proliferation by other cytokines is mandatory in mediating inflammatory responses but the full understanding is far from complete. We have previously reported increased production of IL-2 and IL-2 receptors (IL-2R) in IgA nephropathy. The present study was undertaken to examine other cytokine production during T cell activation in IgA nephropathy. Peripheral blood mononuclear cells (PBMC) from 17 IgA nephritic patients and 14 controls were cultured with phytohaemagglutinin and phorbol myristate acetate for 48 h for maximal cytokine production. IL-2Rs and IL-4 receptors (IL-4Rs) expressed on cultured PBMC were studied by a radioimmunoassay using monoclonal antibodies against these receptors. Although the total cellular IL-2R expression and percentages of T helper and T suppressor cells did not differ between the patients and controls, there was a significant increase in activated T helper cells expressing IL-2R in patients with IgA nephropathy. The total cellular IL-4R expression was elevated in IgA nephritic patients (P less than 0.005). IL-2 production by PBMC was raised in IgA nephritic patients compared with controls (P less than 0.05) but no difference in IL-4 or IL-6 production was observed. The interferon-gamma production by PBMC was significantly increased in patients with IgA nephropathy (P less than 0.025). No correlation was observed between individual cytokine levels. Our data suggest there are selective increases in cytokine production in IgA nephropathy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill F. R., Burke F. The cytokine network. Immunol Today. 1989 Sep;10(9):299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- Billiau A. Interferons and inflammation. J Interferon Res. 1987 Oct;7(5):559–567. doi: 10.1089/jir.1987.7.559. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Ceuppens J. L., Baroja M. L., Lorre K., Van Damme J., Billiau A. Human T cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal. J Immunol. 1988 Dec 1;141(11):3868–3874. [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Aubry J. P., Takebe Y., Arai N., Miyajima A., Yokota T., Lee F., Arai K., de Vries J. E. B cell growth-promoting activity of recombinant human interleukin 4. J Immunol. 1987 Aug 15;139(4):1135–1141. [PubMed] [Google Scholar]
- Deviere J., Content J., Denys C., Vandenbussche P., Schandene L., Wybran J., Dupont E. High interleukin-6 serum levels and increased production by leucocytes in alcoholic liver cirrhosis. Correlation with IgA serum levels and lymphokines production. Clin Exp Immunol. 1989 Aug;77(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause W. C., Takashi T., Mountz J. D., Finkelman F. D., Steinberg A. D. Activation of CD 4-, CD 8- thymocytes with IL 4 vs IL 1 + IL 2. J Immunol. 1988 Oct 1;141(7):2240–2245. [PubMed] [Google Scholar]
- Howard M., Farrar J., Hilfiker M., Johnson B., Takatsu K., Hamaoka T., Paul W. E. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982 Mar 1;155(3):914–923. doi: 10.1084/jem.155.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D. L., Gibert M., Baran D., Ohara J., Paul W. E., Theze J. Activation by IL-2, but not IL-4, up-regulates the expression of the p55 subunit of the IL-2 receptor on IL-2- and IL-4-dependent T cell lines. J Immunol. 1989 May 1;142(9):3113–3120. [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L., Kanagawa O., Brunner K. T. Production of macrophage-activating factor by T lymphocyte clones and correlation with other lymphokine activities. J Immunol. 1982 Aug;129(2):550–556. [PubMed] [Google Scholar]
- Lai K. N., Leung J. C., Lai F. M. In vitro study of expression of interleukin-2 receptors in T-lymphocytes from patients with IgA nephropathy. Clin Nephrol. 1988 Dec;30(6):330–334. [PubMed] [Google Scholar]
- Lai K. N., Leung J. C., Lai F. M., Tam J. S. T-lymphocyte activation in IgA nephropathy: serum-soluble interleukin 2 receptor level, interleukin 2 production, and interleukin 2 receptor expression by cultured lymphocytes. J Clin Immunol. 1989 Nov;9(6):485–492. doi: 10.1007/BF00918018. [DOI] [PubMed] [Google Scholar]
- Larche M., Lamb J. R., O'Hehir R. E., Imami-Shita N., Zanders E. D., Quint D. E., Moqbel R., Ritter M. A. Functional evidence for a monoclonal antibody that binds to the human IL-4 receptor. Immunology. 1988 Dec;65(4):617–622. [PMC free article] [PubMed] [Google Scholar]
- Larché M., Lamb J. R., Ritter M. A. A novel T-lymphocyte molecule that may function in the induction of self-tolerance and MHC-restriction within the human thymic microenvironment. Immunology. 1988 May;64(1):101–105. [PMC free article] [PubMed] [Google Scholar]
- Le J. M., Vilcek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989 Dec;61(6):588–602. [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughnan M. S., Nossal G. J. Interleukins 4 and 5 control expression of IL-2 receptor on murine B cells through independent induction of its two chains. Nature. 1989 Jul 6;340(6228):76–79. doi: 10.1038/340076a0. [DOI] [PubMed] [Google Scholar]
- Maraskovsky E., Chen W. F., Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. J Immunol. 1989 Aug 15;143(4):1210–1214. [PubMed] [Google Scholar]
- Moore R. H., Hitman G. A., Lucas E. Y., Richards N. T., Venning M. C., Papiha S., Goodship T. H., Fidler A., Awad J., Festenstein H. HLA DQ region gene polymorphism associated with primary IgA nephropathy. Kidney Int. 1990 Mar;37(3):991–995. doi: 10.1038/ki.1990.75. [DOI] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Bonnefoy J. Y., Spits H., Yokota T., Arai N., Arai K., Banchereau J. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6880–6884. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F. M., Cochet M. M., Fellous M. Interferon and major histocompatibility complex genes: a model to analyse eukaryotic gene regulation? Interferon. 1986;7:47–87. [PubMed] [Google Scholar]
- Schena F. P., Mastrolitti G., Jirillo E., Munno I., Pellegrino N., Fracasso A. R., Aventaggiato L. Increased production of interleukin-2 and IL-2 receptor in primary IgA nephropathy. Kidney Int. 1989 Mar;35(3):875–879. doi: 10.1038/ki.1989.67. [DOI] [PubMed] [Google Scholar]
- Simon M. M., Hochgeschwender U., Brugger U., Landolfo S. Monoclonal antibodies to interferon-gamma inhibit interleukin 2-dependent induction of growth and maturation in lectin/antigen-reactive cytolytic T lymphocyte precursors. J Immunol. 1986 Apr 15;136(8):2755–2762. [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Yssel H., Takebe Y., Arai N., Yokota T., Lee F., Arai K., Banchereau J., de Vries J. E. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987 Aug 15;139(4):1142–1147. [PubMed] [Google Scholar]
- Vilcek J., Henriksen-Destefano D., Siegel D., Klion A., Robb R. J., Le J. Regulation of IFN-gamma induction in human peripheral blood cells by exogenous and endogenously produced interleukin 2. J Immunol. 1985 Sep;135(3):1851–1856. [PubMed] [Google Scholar]
- Widmer M. B., Grabstein K. H. Regulation of cytolytic T-lymphocyte generation by B-cell stimulatory factor. Nature. 1987 Apr 23;326(6115):795–798. doi: 10.1038/326795a0. [DOI] [PubMed] [Google Scholar]


