Abstract
Histopathological studies have provided circumstantial evidence that helminth parasite destruction occurs in the lung; however controlled in vitro studies on the helminthocidal activity of lung cells have not been reported. This study presents evidence that Nippostrongylus brasiliensis infection in the rat induces alterations in broncho-alveolar lavage (BAL) cell numbers, differential counts, and in vitro helminthocidal activity. Normal, uninfected rats yielded 3.3 +/- 0.6 X 10(6) BAL cells/rat, consisting predominantly of alveolar macrophages (greater than 90%). However on days 2-8 post-infection there was a 1.5-2.4-fold increase in BAL cell numbers with a significant neutrophilia on day 2 and a significant increase in the absolute number of all cell types on day 8. On day 32 post-infection, BAL cell numbers had returned to control levels. Normal BAL cells neither adhered to nor killed N. brasiliensis infective larvae (L3) in the presence of rat complement. By contrast BAL cells recovered from infected rats on days, 2, 8 or 32 post-infection (D2, D8 and D32 BAL cells, respectively) adhered under similar conditions. However, only D8 and D32 BAL cells killed L3. This complement-dependent killing correlated with significantly increased numbers of C3 receptor bearing alveolar macrophages in D8 and D32 BAL cells. Complement-dependent alveolar macrophage helminthocidal activity may therefore play an important role in lung resistance against resident or migrating helminths.
Full text
PDF


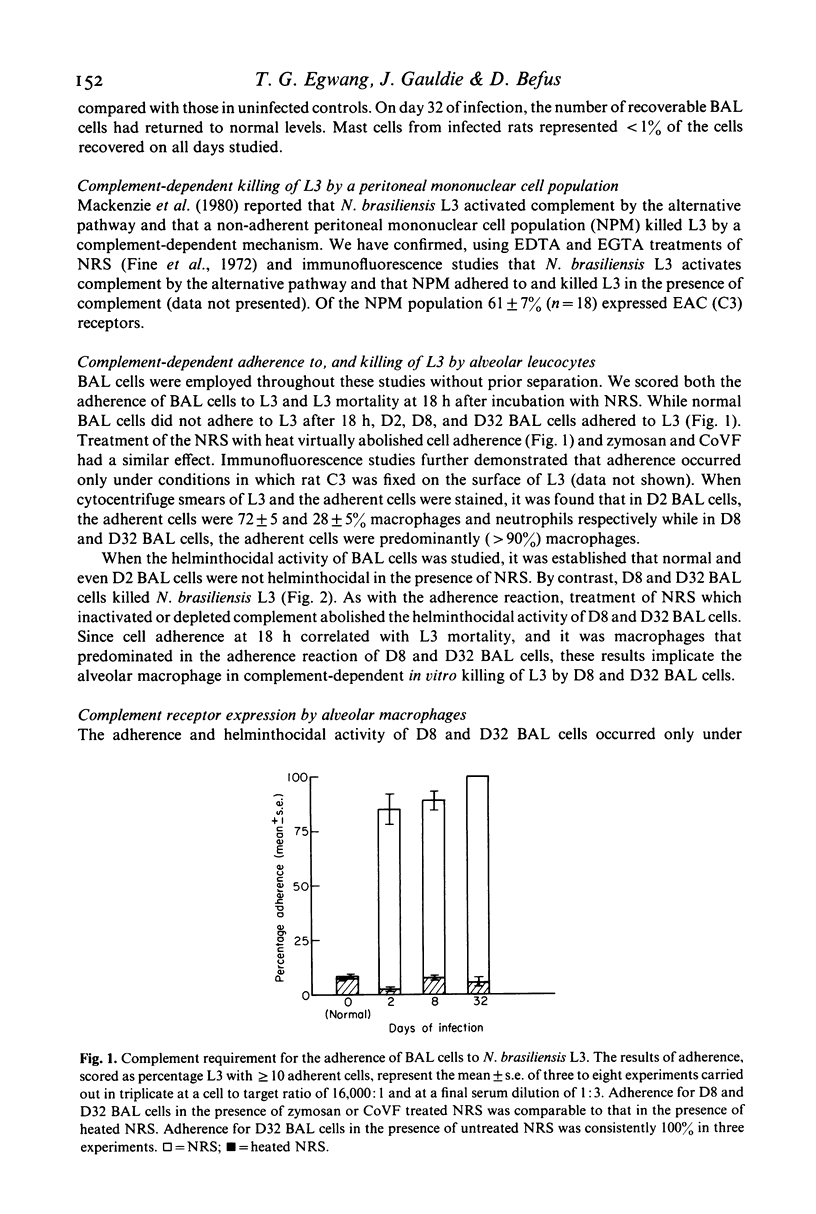
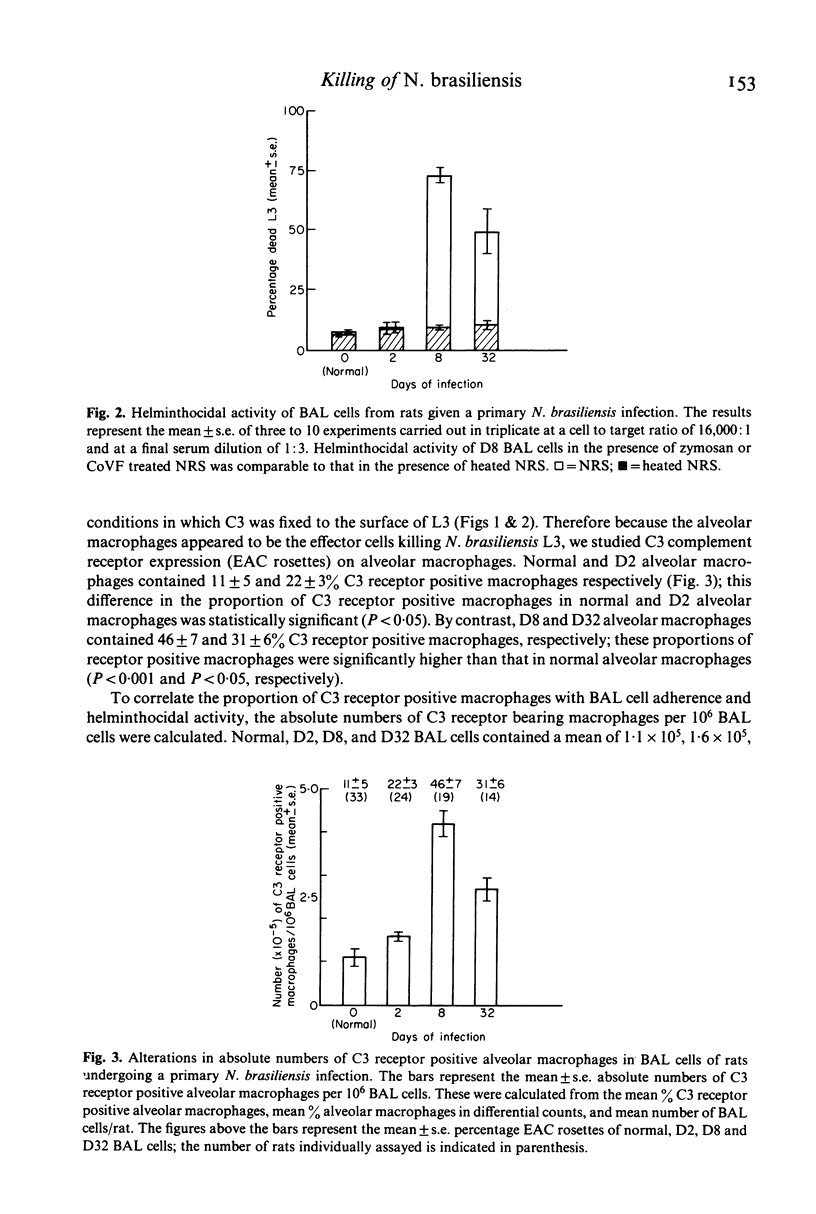



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ades E. W., Hinson A., Chapuis-Cellier C., Arnaud P. Modulation of the immune response by plasma protease inhibitors. I. Alpha 2-macroglobulin and alpha 1-antitrypsin inhibit natural killing and antibody-dependent cell-mediated cytotoxicity. Scand J Immunol. 1982 Jan;15(1):109–113. doi: 10.1111/j.1365-3083.1982.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Szejda P. Mechanisms of killing of newborn larvae of Trichinella spiralis by neutrophils and eosinophils. Killing by generators of hydrogen peroxide in vitro. J Clin Invest. 1979 Dec;64(6):1558–1564. doi: 10.1172/JCI109616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer M. H., Spry C. J., Beeson P. B., Sheldon W. H. Mechanism of eosinophilia. IV. The pulmonary lesion resulting from intravenous injection of Trichinella spiralis. Yale J Biol Med. 1971 Jun;43(6):351–357. [PMC free article] [PubMed] [Google Scholar]
- Brain J. D., Frank N. R. Recovery of free cells from rat lungs by repeated washings. J Appl Physiol. 1968 Jul;25(1):63–69. doi: 10.1152/jappl.1968.25.1.63. [DOI] [PubMed] [Google Scholar]
- Capron A., Dessaint J. P., Capron M., Bazin H. Specific IgE antibodies in immune adherence of normal macrophages to Schistosoma mansoni schistosomules. Nature. 1975 Feb 6;253(5491):474–475. doi: 10.1038/253474a0. [DOI] [PubMed] [Google Scholar]
- DAVIS J. R., HSU S. Y., HSU H. F. Comparative histopathological study on Schistosoma japonicum infection in immunized and non-immunized rhesus monkeys. Z Tropenmed Parasitol. 1963 Apr;14:21–36. [PubMed] [Google Scholar]
- Dean D. A., Wistar R., Murrell K. D. Combined in vitro effects of rat antibody and neutrophilic leukocytes on schistosomula of Schistosoma mansoni. Am J Trop Med Hyg. 1974 May;23(3):420–428. doi: 10.4269/ajtmh.1974.23.420. [DOI] [PubMed] [Google Scholar]
- Dierich M. P., Landen B., Schmitt M. Complement receptor analogous factors in human serum: I. Isolation of a molecule inhibitory for complement dependent rosette formation, its identification as alpha 1-antitrypsin and its functional characterization. Immunobiology. 1979 Aug;156(1-2):153–167. [PubMed] [Google Scholar]
- Ellner J. J., Olds G. R., Lee C. W., Kleinhenz M. E., Edmonds K. L. Destruction of the multicellular parasite Schistosoma mansoni by T lymphocytes. J Clin Invest. 1982 Aug;70(2):369–378. doi: 10.1172/JCI110626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Kawanami O., Ferrans V. J., Young R. C., Jr, Roberts W. C., Crystal R. G. Characterization of the inflammatory and immune effector cells in the lung parenchyma of patients with interstitial lung disease. Am Rev Respir Dis. 1981 Apr;123(4 Pt 1):407–412. doi: 10.1164/arrd.1981.123.4.407. [DOI] [PubMed] [Google Scholar]
- JARRETT W. F., SHARP N. C. Vaccination against parasitic disease: reactions in vaccinated and immune host in Dictovacaulus viviparus infection. J Parasitol. 1963 Apr;49:177–189. [PubMed] [Google Scholar]
- Kazura J. W., Fanning M. M., Blumer J. L., Mahmoud A. A. Role of cell-generated hydrogen peroxide in granulocyte-mediated killing of schistosomula of Schistosoma mansoni in vitro. J Clin Invest. 1981 Jan;67(1):93–102. doi: 10.1172/JCI110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love R. J., Ogilvie B. M. Nippostrongylus brasiliensis in young rats. Lymphocytes expel larval infections but not adult worms. Clin Exp Immunol. 1975 Jul;21(1):155–162. [PMC free article] [PubMed] [Google Scholar]
- Mackenzie C. D., Jungery M., Taylor P. M., Ogilvie B. M. Activation of complement, the induction of antibodies to the surface of nematodes and the effect of these factors and cells on worm survival in vitro. Eur J Immunol. 1980 Aug;10(8):594–601. doi: 10.1002/eji.1830100805. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Peters P. A., Civil R. H., Remington J. S. In vitro killing of schistosomula of Schistosoma mansoni by BCG and C. parvum-activated macrophages. J Immunol. 1979 May;122(5):1655–1657. doi: 10.2196/41502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren D. J., Ramalho-Pinto F. J. Eosinophil-mediated killing of schistosomula of Schistosoma mansoni in vitro: synergistic effect of antibody and complement. J Immunol. 1979 Oct;123(4):1431–1438. [PubMed] [Google Scholar]
- Mód A., Füst G., Gergely J., Hollán S. R., Dierich M. P. Alpha-1-antitrypsin-induced inhibition of complement-dependent phagocytosis. Immunobiology. 1981;158(4):338–346. doi: 10.1016/S0171-2985(81)80005-8. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Ellner J. J., Kearse L. A., Jr, Kazura J. W., Mahmoud A. A. Role of arginase in killing of schistosomula of Schistosoma mansoni. J Exp Med. 1980 Jun 1;151(6):1557–1562. doi: 10.1084/jem.151.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryning F. W., Krahenbuhl J. L., Remington J. S. Comparison of cytotoxic and microbicidal function of bronchoalveolar and peritoneal macrophages. Immunology. 1981 Apr;42(4):513–519. [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Secretion of a specific collagenase by stimulated macrophages. J Exp Med. 1975 Aug 1;142(2):346–360. doi: 10.1084/jem.142.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lichtenberg F., Sher A., McIntyre S. A lung model of schistosome immunity in mice. Am J Pathol. 1977 Apr;87(1):105–123. [PMC free article] [PubMed] [Google Scholar]


