Abstract
We examined, in a 'double blind' study, 60 sera from patients with pernicious anaemia for immunofluorescence reactivity with the surface membranes of viable parietal cells isolated from dog stomachs. Fifty-three sera (88%) gave an IgG autoantibody reaction with the surface membranes of parietal cells. Surface staining was also seen with parietal cells from monkey, pig, rat and mouse. The parietal cell surface reactive autoantibody was not found in any of 14 sera from patients with chronic active hepatitis, 10 from patients with systemic lupus erythematosus and 50 from healthy persons. The surface reactivity autoantibody was present in 13 of 14 sera without parietal cell microsomal antibody, 28 of 31 sera without intrinsic factor antibody and in four of four sera without microsomal and intrinsic factor antibodies. Absorption with parietal cell enriched gastric mucosal cells neutralized the activity of the surface reactive but not the microsomal antibody and cross absorption with gastric microsomes neutralized the activity of the microsomal but not the surface reactive antibody. Surface staining of parietal cells was not abolished by absorption with dog or rat hepatocytes, dog or rat kidney cells, human fibroblasts or human AB red blood cells. The results suggest that the parietal cell surface reactive antibody is probably different from the microsomal antibody. Immune reactions of the cell surface reactive antibody with parietal cell surface antigens may play a role in the pathogenesis of the gastric lesion in pernicious anaemia.
Full text
PDF

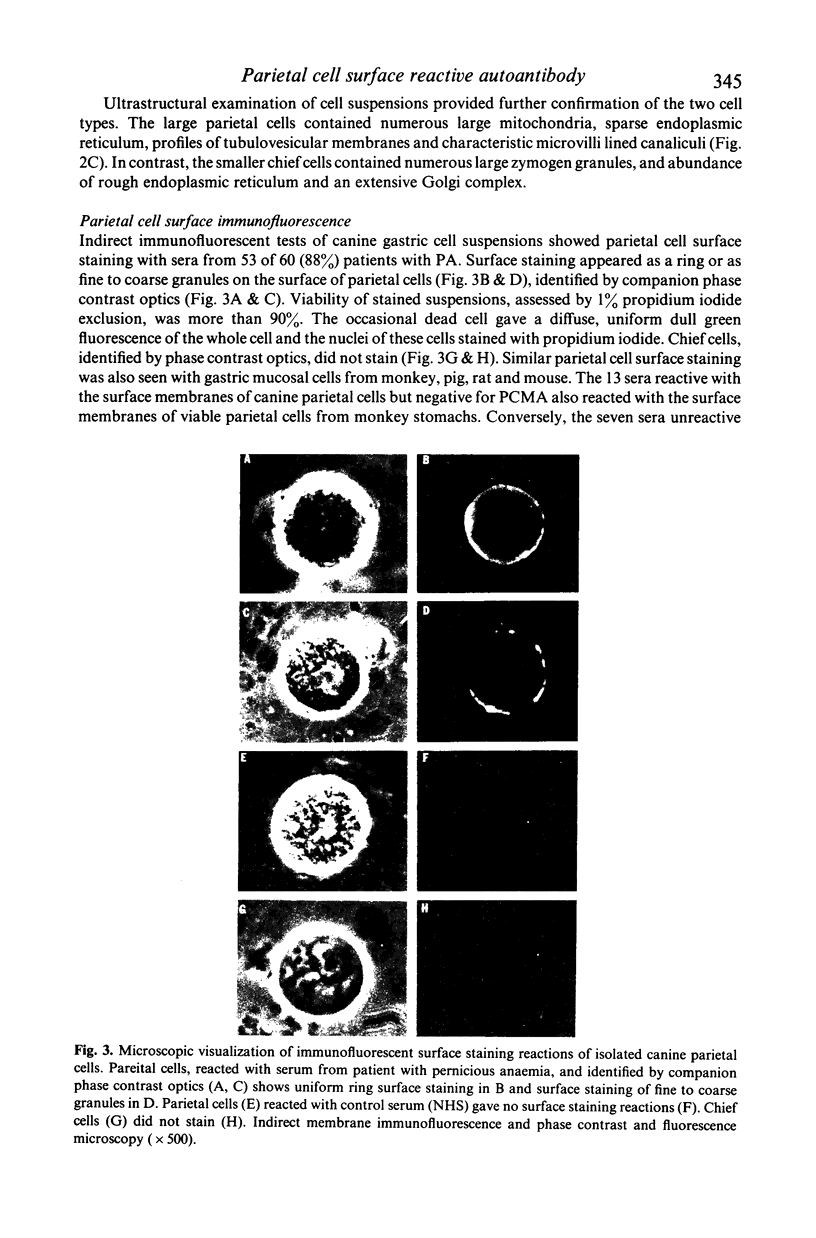
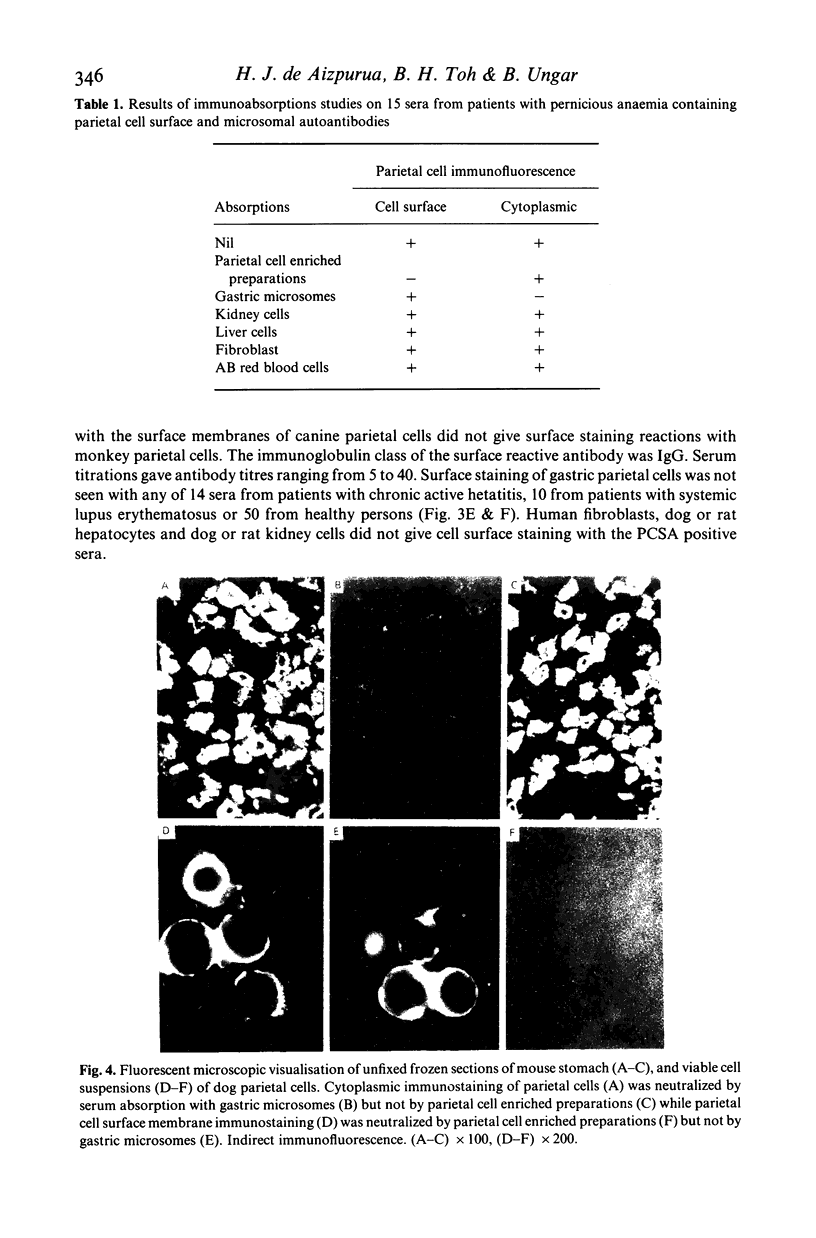



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carnegie P. R., Mackay I. R. Vulnerability of cell-surface receptors to autoimmune reactions. Lancet. 1975 Oct 11;2(7937):684–687. doi: 10.1016/s0140-6736(75)90779-5. [DOI] [PubMed] [Google Scholar]
- Ceredig R., Toh B. H. Ontogeny of actin and microsomal antigens in gastric parietal cells. J Clin Pathol. 1978 Jun;31(6):578–584. doi: 10.1136/jcp.31.6.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Morris M. A., Scearce R. M. Cytotoxic antibodies to cloned rat islet cells in serum of patients with diabetes mellitus. J Clin Invest. 1981 Feb;67(2):403–408. doi: 10.1172/JCI110048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoedemaeker P. J., Ito S. Ultrastructural localization of gastric parietal cell antigen with peroxidase-coupled antibody. Lab Invest. 1970 Feb;22(2):184–188. [PubMed] [Google Scholar]
- Hopf U., Meyer zum Büschenfelde K. H., Arnold W. Detection of a liver-membrane autoantibody in HBsAg-negative chronic active hepatitis. N Engl J Med. 1976 Mar 11;294(11):578–582. doi: 10.1056/NEJM197603112941103. [DOI] [PubMed] [Google Scholar]
- JEFFRIES G. H., HOSKINS D. W., SLEISENGER M. H. Antibody to intrinsic factor in serum from patients with pernicious anemia. J Clin Invest. 1962 May;41:1106–1115. doi: 10.1172/JCI104562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. R. The trophic action of gastrointestinal hormones. Gastroenterology. 1976 Feb;70(2):278–288. [PubMed] [Google Scholar]
- Khoury E. L., Hammond L., Bottazzo G. F., Doniach D. Presence of the organ-specific 'microsomal' autoantigen on the surface of human thyroid cells in culture: its involvement in complement-mediated cytotoxicity. Clin Exp Immunol. 1981 Aug;45(2):316–328. [PMC free article] [PubMed] [Google Scholar]
- Lernmark A., Freedman Z. R., Hofmann C., Rubenstein A. H., Steiner D. F., Jackson R. L., Winter R. J., Traisman H. S. Islet-cell-surface antibodies in juvenile diabetes mellitus. N Engl J Med. 1978 Aug 24;299(8):375–380. doi: 10.1056/NEJM197808242990802. [DOI] [PubMed] [Google Scholar]
- Loveridge N., Bitensky L., Chayen J., Hausamen T. U., Fisher J. M., Taylor K. B., Gardner J. D., Bottazzo G. F., Doniach D. Inhibition of parietal cell function by human gammaglobulin containing gastric parietal cell antibodies. Clin Exp Immunol. 1980 Aug;41(2):264–270. [PMC free article] [PubMed] [Google Scholar]
- Masala C., Smurra G., Di Prima M. A., Amendolea M. A., Celestino D., Salsano F. Gastric parietal cell antibodies: demonstration by immunofluorescence of their reactivity with surface of the gastric parietal cells. Clin Exp Immunol. 1980 Aug;41(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- Meek F., Khoury E. L., Doniach D., Baum H. Mitochondrial antibodies in chronic liver diseases and connective tissue disorders: further characterization of the autoantigens. Clin Exp Immunol. 1980 Jul;41(1):43–54. [PMC free article] [PubMed] [Google Scholar]
- Nairn R. C., Rolland J. M. Fluorescent probes to detect lymphocyte activation. Clin Exp Immunol. 1980 Jan;39(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Romrell L. J., Coppe M. R., Munro D. R., Ito S. Isolation and separation of highly enriched fractions of viable mouse gastric parietal cells by velocity sedimentation. J Cell Biol. 1975 May;65(2):428–438. doi: 10.1083/jcb.65.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumarmon A., Cheret A. M., Lewin M. J. Localization of gastrin receptors in intact isolated and separated rat fundic cells. Gastroenterology. 1977 Oct;73(4 Pt 2):900–903. [PubMed] [Google Scholar]
- Strickland R. G., Mackay I. R. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973 May;18(5):426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- TAYLOR K. B., ROITT I. M., DONIACH D., COUCHMAN K. G., SHAPLAND C. Autoimmune phenomena in pernicious anaemia: gastric antibodies. Br Med J. 1962 Nov 24;2(5316):1347–1352. doi: 10.1136/bmj.2.5316.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar B., Francis C. M., Cowling D. C. Antibody to parietal cells in patients with duodenal ulcer, and relationship to pernicious anaemia. Med J Aust. 1976 Dec 11;2(24):900–902. doi: 10.5694/j.1326-5377.1976.tb115487.x. [DOI] [PubMed] [Google Scholar]
- Ungar B., Mathews J. D., Tait B. D., Cowling D. C. HLA-DR patterns in pernicious anaemia. Br Med J (Clin Res Ed) 1981 Mar 7;282(6266):768–770. doi: 10.1136/bmj.282.6266.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar B., Stocks A. E., Martin F. I., Whittingham S., Mackay I. R. Intrinsic-factor antibody, parietal-cell antibody, and latent pernicious anaemia in diabetes mellitus. Lancet. 1968 Aug 24;2(7565):415–417. doi: 10.1016/s0140-6736(68)90462-5. [DOI] [PubMed] [Google Scholar]