Abstract
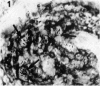
Full thickness skin biopsies from four patients with borderline lepromatous leprosy (BL leprosy) have been examined. Immunohistological techniques have been employed to analyse the non-lymphoid mononuclear cells present in the dermal infiltrates associated with the BL lesions. This analysis was performed using three monoclonal antibodies, RFD2 (recognizing macrophages), RFD1 (recognizing interdigitating cells) and NA1/34 (recognizing Langerhans cells). It was found that the vast majority of non-lymphoid mononuclear cells in the lesions were RFD2+ macrophages. However, a significant number (15-30%) of macrophage like cells were RFD1+ interdigitating cells. A very small number of NA1/34+ Langerhans cells were also identified within the dermal infiltrates. Combination immunohistology and Ziehl Neelsen staining revealed that all these cell types could be found containing the Mycobacterium leprae organisms. The proportions of parasitized cells within each subpopulation was equivalent to the overall proportion of each cell type within the infiltrate. The significance of parasitism of cell types thought to be involved in antigen presentation and induction of immune responses is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balfour B. M., Drexhage H. A., Kamperdijk E. W., Hoefsmit E. C. Antigen-presenting cells, including Langerhans cells, veiled cells and interdigitating cells. Ciba Found Symp. 1981;84:281–301. doi: 10.1002/9780470720660.ch15. [DOI] [PubMed] [Google Scholar]
- Convit J., Pinardi M. E., Rodríguez Ochoa G., Ulrich M., Avila J. L., Goihman M. Elimination of Mycobacterium leprae subsequent to local in vivo activation of macrophages in lepromatous leprosy by other mycobacteria. Clin Exp Immunol. 1974 Jun;17(2):261–265. [PMC free article] [PubMed] [Google Scholar]
- Humphrey J. H. Differentiation of function among antigen-presenting cells. Ciba Found Symp. 1981;84:302–321. doi: 10.1002/9780470720660.ch16. [DOI] [PubMed] [Google Scholar]
- Janossy G., Thomas J. A., Goldstein G., Bollum F. J. The human thymic environment. Ciba Found Symp. 1981;84:193–214. [PubMed] [Google Scholar]
- Mishra B. B., Poulter L. W., Janossy G., James D. G. The distribution of lymphoid and macrophage like cell subsets of sarcoid and Kveim granulomata: possible mechanism of negative PPD reaction in sarcoidosis. Clin Exp Immunol. 1983 Dec;54(3):705–715. [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Taylor C. R., Rea T. H. T lymphocyte subsets in the skin lesions of patients with leprosy. J Am Acad Dermatol. 1983 Feb;8(2):182–189. doi: 10.1016/s0190-9622(83)70021-6. [DOI] [PubMed] [Google Scholar]
- Narayanan R. B., Bhutani L. K., Sharma A. K., Nath I. T cell subsets in leprosy lesions: in situ characterization using monoclonal antibodies. Clin Exp Immunol. 1983 Mar;51(3):421–429. [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Gutchinov B., Cohn Z. A. Dendritic cells are accessory cells for the development of anti-trinitrophenyl cytotoxic T lymphocytes. J Exp Med. 1980 Oct 1;152(4):1070–1084. doi: 10.1084/jem.152.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W. Antigen presenting cells in situ: their identification and involvement in immunopathology. Clin Exp Immunol. 1983 Sep;53(3):513–520. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Duke O., Hobbs S., Janossy G., Panayi G., Seymour G. The involvement of interdigitating (antigen-presenting) cells in the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 1983 Feb;51(2):247–254. [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Seymour G. J., Duke O., Janossy G., Panayi G. Immunohistological analysis of delayed-type hypersensitivity in man. Cell Immunol. 1982 Dec;74(2):358–369. doi: 10.1016/0008-8749(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Silberberg-Sinakin I., Thorbecke G. J., Baer R. L., Rosenthal S. A., Berezowsky V. Antigen-bearing langerhans cells in skin, dermal lymphatics and in lymph nodes. Cell Immunol. 1976 Aug;25(2):137–151. doi: 10.1016/0008-8749(76)90105-2. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973 May 1;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]