Abstract
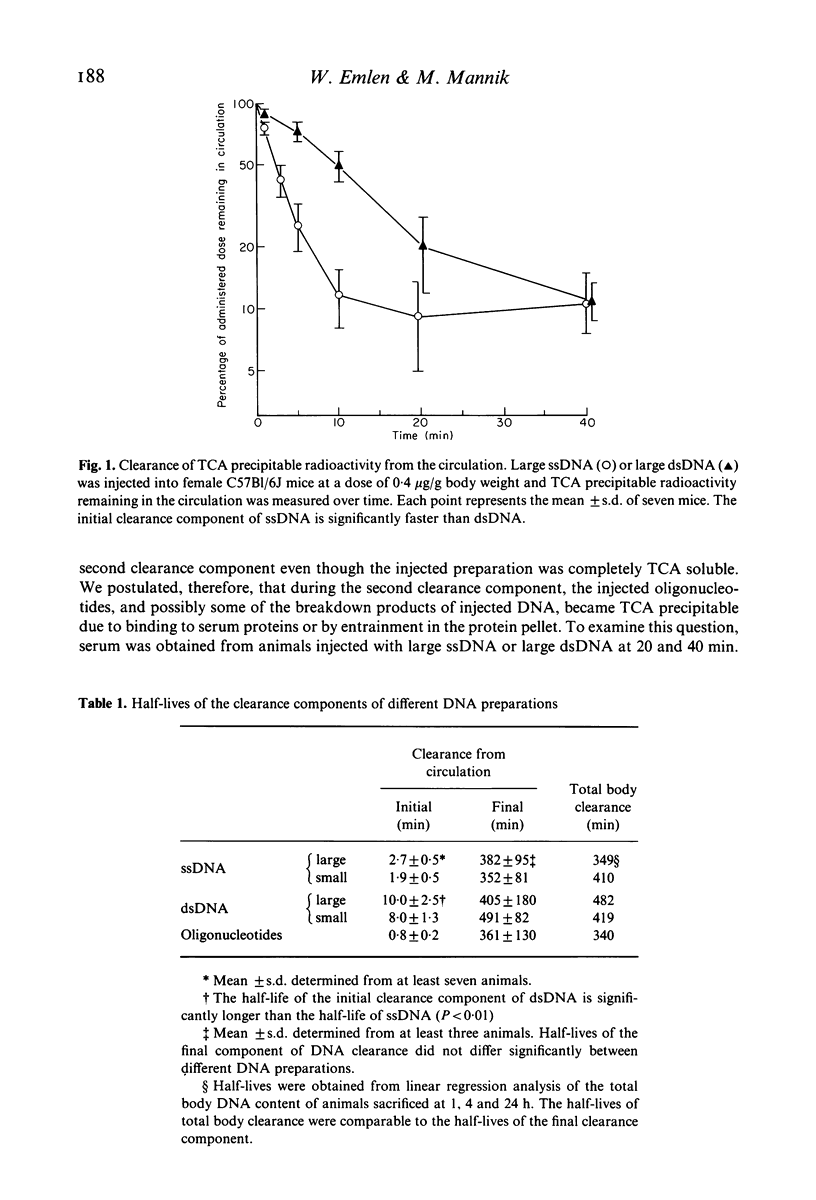
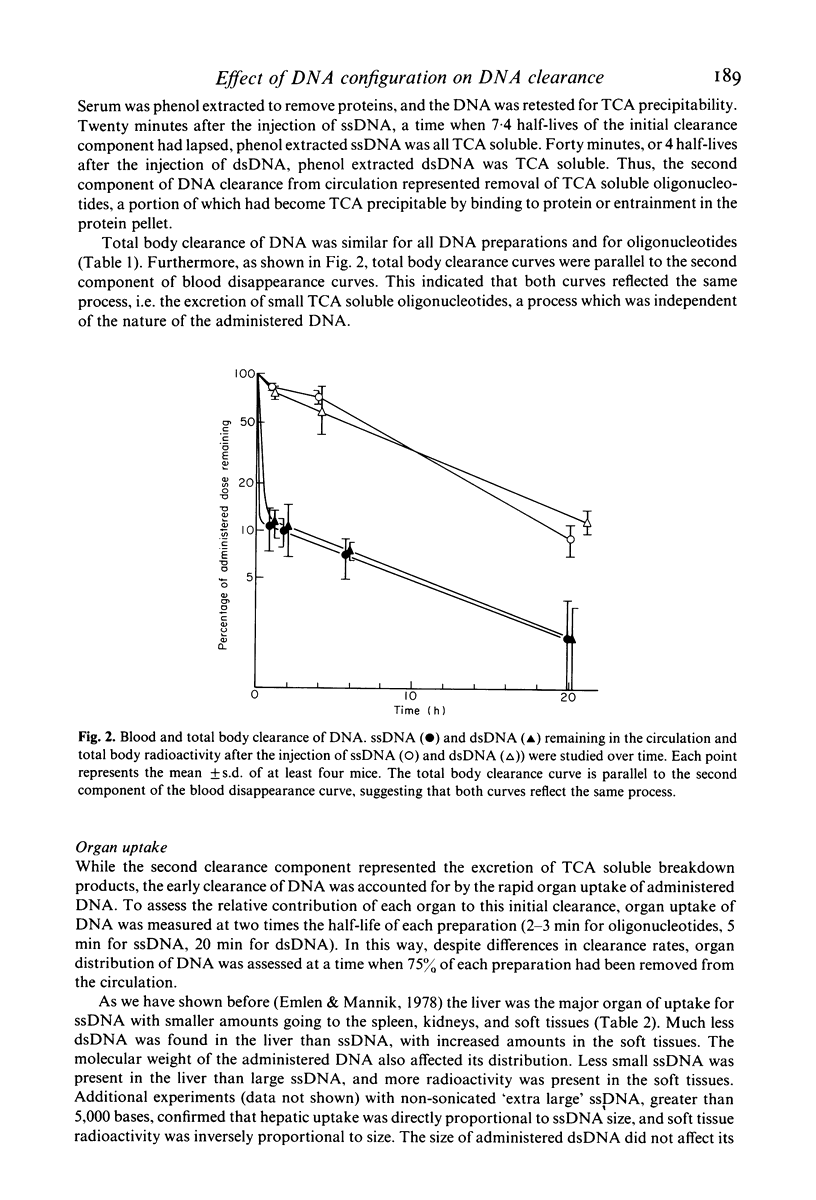
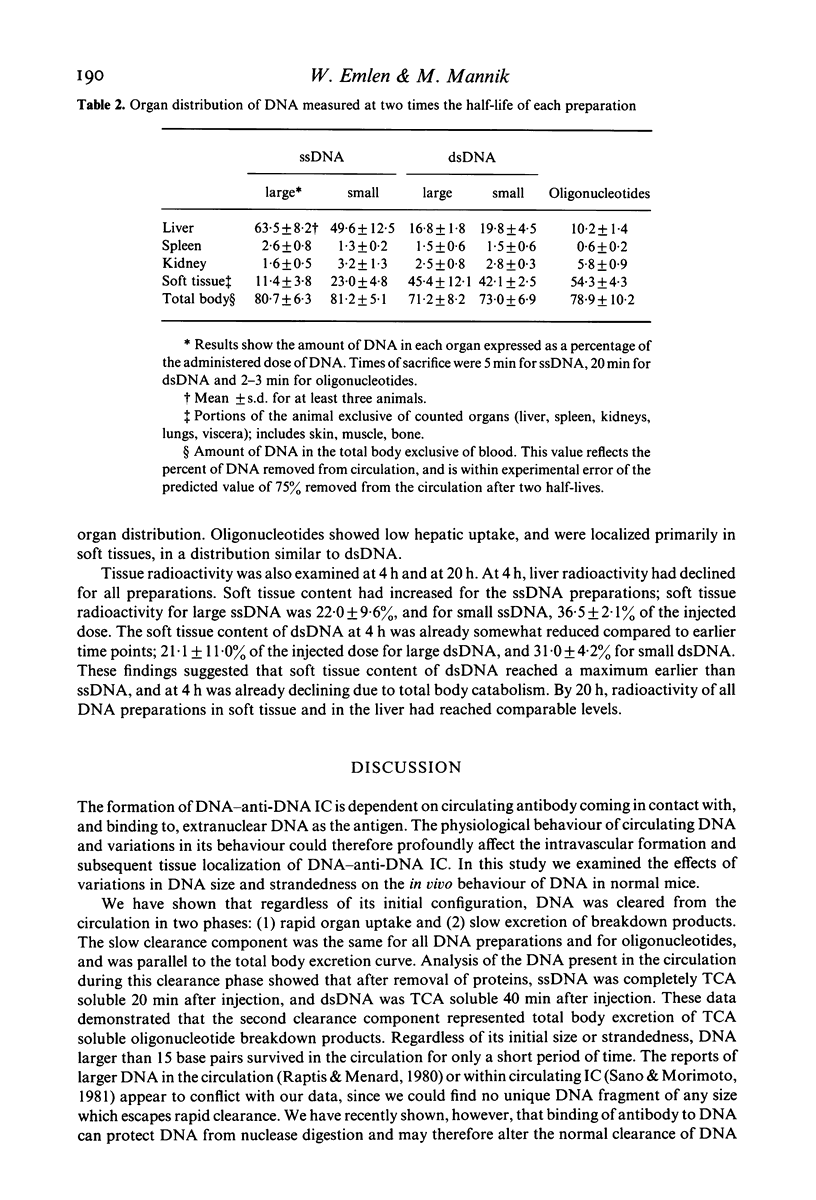
DNA-anti-DNA immune complexes play a major role in the pathogenesis of SLE. Evidence suggests that the DNA contained within these complexes, as well as free circulating DNA, is of small molecular weight and predominantly double stranded. Previous studies have shown that large DNA is cleared from circulation rapidly and efficiently. To examine if variations in the configuration of DNA itself affected its ability to persist in the circulation, we studied the clearance and organ uptake of single stranded DNA(ssDNA) and double stranded DNA(dsDNA) of different sizes in normal mice. Clearance of DNA from the circulation was described by two exponential components. The first component represented organ uptake, and was much more rapid for ssDNA than for dsDNA. The second component represented the excretion of breakdown products from the total body pool, and was the same for all DNA preparations. Regardless of its initial size, DNA larger than 15 bases did not persist in the circulation longer than 20 min for ssDNA, and longer than 40 min for dsDNA. Organ distribution studies showed that ssDNA was removed by the liver, but that dsDNA bound poorly to the liver and was distributed like oligonucleotide breakdown products. Our results suggest that dsDNA and ssDNA are removed from the circulation by different mechanisms. Although dsDNA remains in the circulation slightly longer than ssDNA, all DNA, regardless of its size or strandedness, is cleared from the circulation and broken down rapidly and efficiently.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. S., Oakley C. L., Rowell N. R. Transplacental passage of antinuclear antibody. Study in infants of mothers with systemic lupus erythematosus. Arch Dermatol. 1966 Jun;93(6):656–663. doi: 10.1001/archderm.93.6.656. [DOI] [PubMed] [Google Scholar]
- Chused T. M., Steinberg A. D., Talal N. The clearance and localization of nucleic acids by New Zealand and normal mice. Clin Exp Immunol. 1972 Dec;12(4):465–476. [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Boyer H. W. Solubility and dialysis limits of DNA oligonucleotides. Biochim Biophys Acta. 1972 Mar 14;262(2):116–124. doi: 10.1016/0005-2787(72)90224-9. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Dorsch C. A., Chia D., Levy L., Barnett E. V. Persistence of DNA in the circulation of immunized rabbits. J Rheumatol. 1975 Jun;2(2):161–166. [PubMed] [Google Scholar]
- Emlen W., Mannik M. Effect of preformed immune complexes on the clearance and tissue localization of single-stranded DNA in mice. Clin Exp Immunol. 1980 May;40(2):264–272. [PMC free article] [PubMed] [Google Scholar]
- Emlen W., Mannik M. Kinetics and mechanisms for removal of circulating single-stranded DNA in mice. J Exp Med. 1978 Mar 1;147(3):684–699. doi: 10.1084/jem.147.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournie G. J., Izui S., Lambert P. H., Conte J. J. Genesis and pathogenicity of anti-DNA antibodies. Adv Nephrol Necker Hosp. 1976;6:47–61. [PubMed] [Google Scholar]
- Frost P. G., Lachmann P. J. The relationship of desoxyribonuclease inhibitor levels in human sera to the occurrence of antinuclear antibodies. Clin Exp Immunol. 1968 Jun;3(5):447–455. [PMC free article] [PubMed] [Google Scholar]
- Izui S., Lambert P. H., Miescher P. A. In vitro demonstration of a particular affinity of glomerular basement membrane and collagen for DNA. A possible basis for a local formation of DNA-anti-DNA complexes in systemic lupus erythematosus. J Exp Med. 1976 Aug 1;144(2):428–443. doi: 10.1084/jem.144.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffler D., Carr R., Agnello V., Thoburn R., Kunkel H. G. Antibodies to polynucleotides in human sera: antigenic specificity and relation to disease. J Exp Med. 1971 Jul 1;134(1):294–312. doi: 10.1084/jem.134.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J. D., Medof M. E., Bennett R. M., Sukhupunyaraksa S. Characterization of DNA used to assay sera for anti-DNA antibodies; determination of the specificities of anti-DNA antibodies in SLE and non-SLE rheumatic disease states. J Immunol. 1977 Feb;118(2):694–701. [PubMed] [Google Scholar]
- Love J. D., Hewitt R. R. The relationship between human serum and human pancreatic DNase I. J Biol Chem. 1979 Dec 25;254(24):12588–12594. [PubMed] [Google Scholar]
- Pancer L. B., Milazzo M. F., Morris V. L., Singhal S. K., Bell D. A. Immunogenicity and characterization of supernatant DNA released by murine spleen cells. J Immunol. 1981 Jul;127(1):98–104. [PubMed] [Google Scholar]
- Raptis L., Menard H. A. Quantitation and characterization of plasma DNA in normals and patients with systemic lupus erythematosus. J Clin Invest. 1980 Dec;66(6):1391–1399. doi: 10.1172/JCI109992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Morimoto C. Isolation of DNA from DNA/anti-DNA antibody immune complexes in systemic lupus erythematosus. J Immunol. 1981 Feb;126(2):538–539. [PubMed] [Google Scholar]
- Tan E. M., Schur P. H., Carr R. I., Kunkel H. G. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1966 Nov;45(11):1732–1740. doi: 10.1172/JCI105479. [DOI] [PMC free article] [PubMed] [Google Scholar]


