Abstract
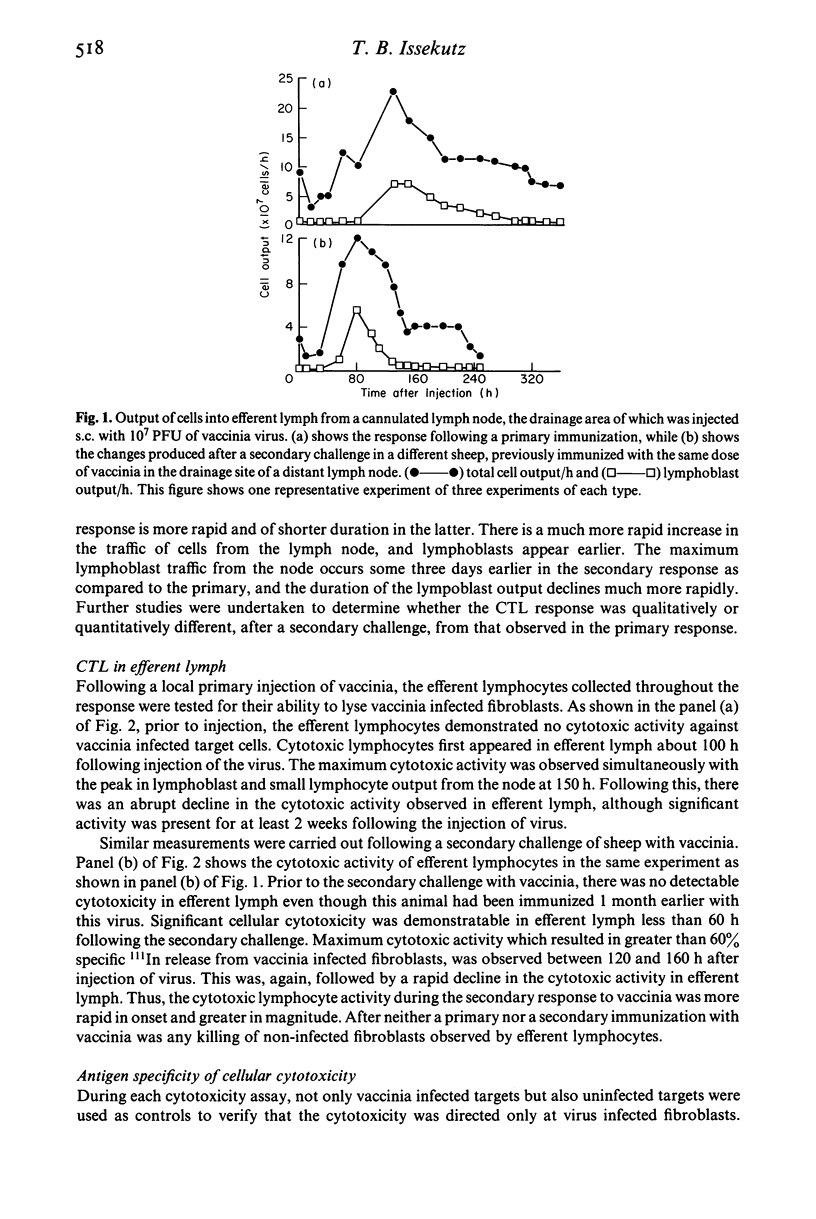
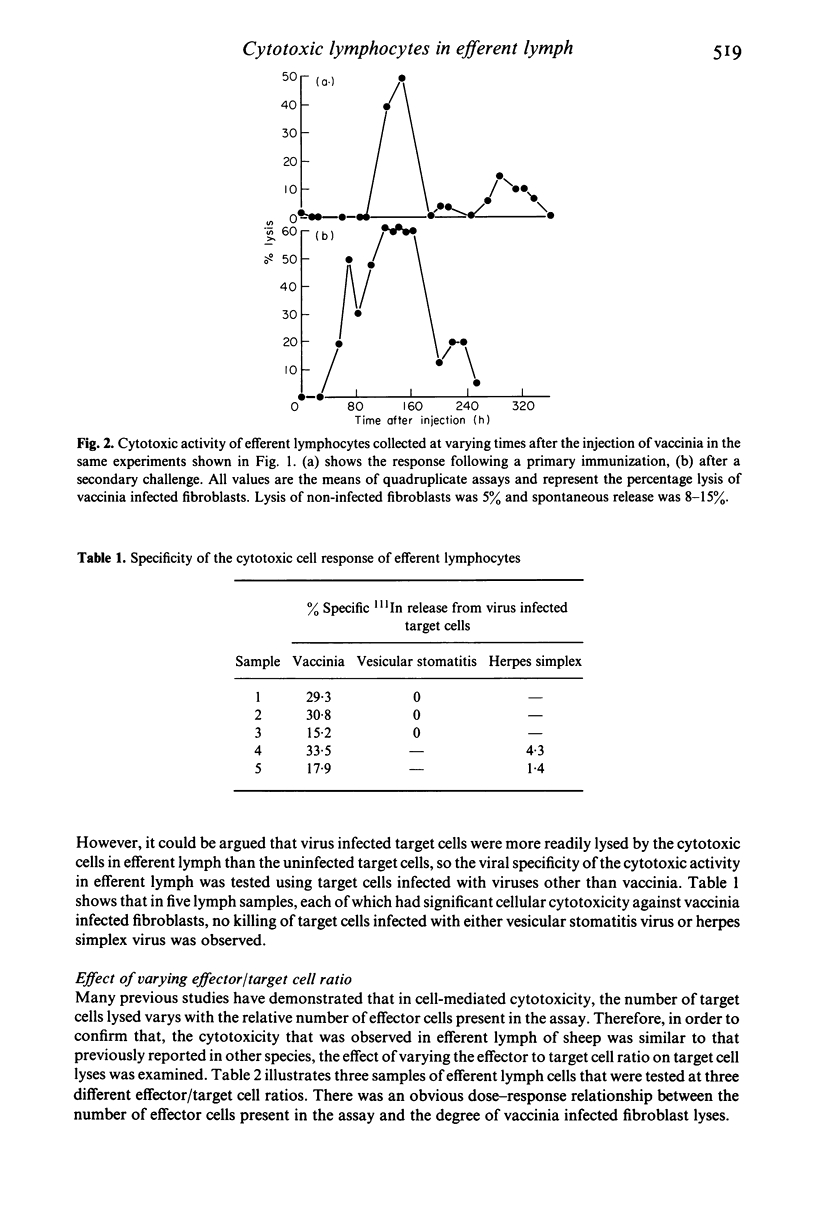
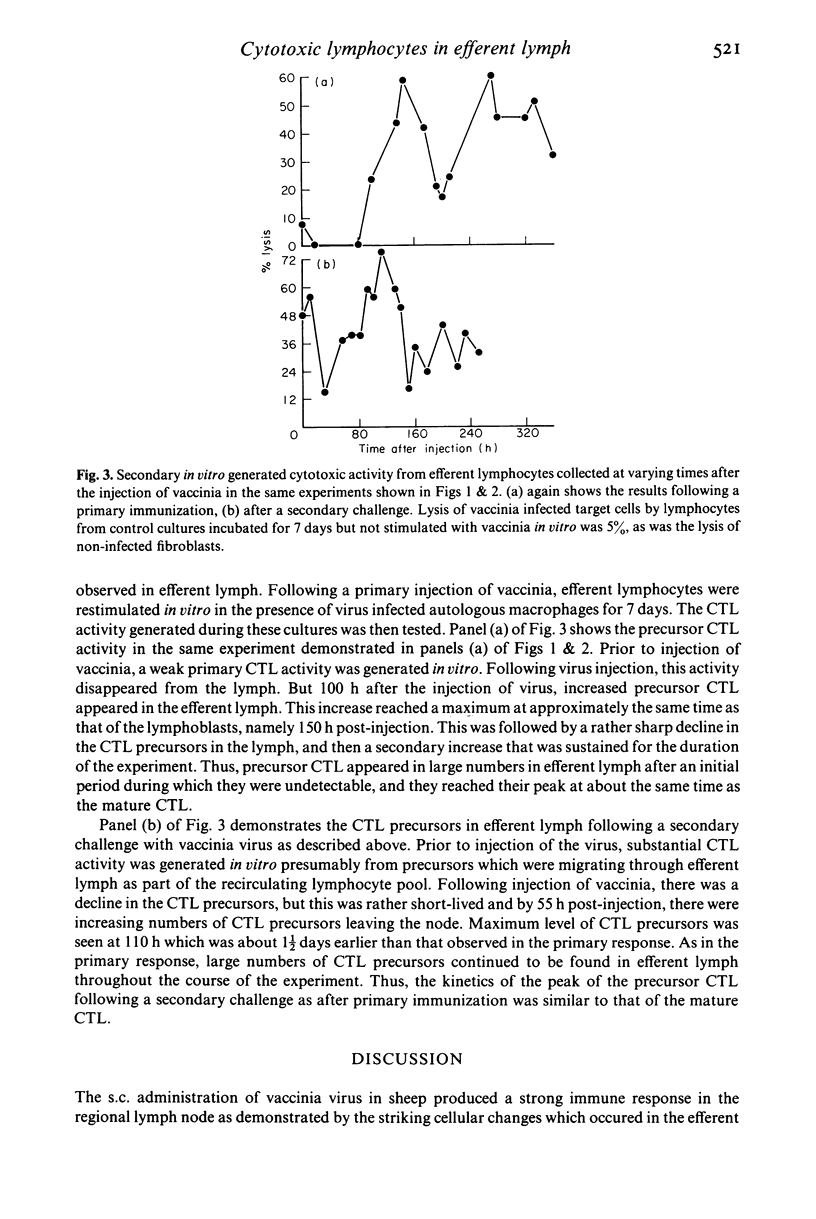
Efferent lymphocytes collected from a cannulated lymphatic draining single lymph nodes were studied for their cytotoxic activity following the injection of live vaccinia virus subcutaneously into the drainage site of a lymph node. Injection of virus produced a 40-fold increase in the lymphoblast output 7 days following virus injection. Cytotoxic lymphocytes were detectable in lymph shortly after the appearance of lymphoblasts at 80 h and also reached a maximum during the 7th day. This was followed by a rapid decline of the cytotoxic cells although cytotoxic cells were detectable up to 2 weeks. The cytotoxic activity in lymph was found to be antigen specific, dependent on the effector/target cell ratio, and allogeneically restricted, indicating that it was most likely due to cytotoxic T lymphocytes (CTL). CTL precursors were found in large numbers in efferent lymph and appeared at approximately the same time as the mature CTL. Unlike CTL, the precursors became part of the recirculating lymphocyte pool and were detectable in efferent lymph for at least 2 months. Following a secondary challenge with vaccinia, lymphoblasts and CTL appeared at least 36 h earlier in the lymph. In summary, we have demonstrated that virus specific CTL are found in the efferent lymph collected from a single immunized lymph node in sheep. The kinetics of the CTL and CTL precursors indicate that these lymphocytes are one of the earliest antigen specific cells detectable in efferent lymph and suggests that these cells migrate rapidly from the lymph node into efferent lymph for dissemination throughout the host to sites of virus infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denham S., Hall J. G., Wolf A., Alexander P. The nature of the cytotoxic cells in lymph following primary antigenic challenge. Transplantation. 1969 Mar;7(3):194–203. doi: 10.1097/00007890-196903000-00006. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M., Ramshaw I. A. Specificity and development of cytotoxic thymus-derived lymphocytes in lymphocytic choriomeningitis. J Immunol. 1974 Apr;112(4):1548–1552. [PubMed] [Google Scholar]
- Grant C. K., Avis P. J., Hall J. G., Alexander P. Evidence for the role of antibody in the cytotoxic action of lymph cells on xenogeneic target cells. Transplantation. 1971 Mar;11(3):356–358. [PubMed] [Google Scholar]
- Grant C. K., Denham S., Hall J. G., Alexander P. Antibody and complement-like factors in the cytotoxic action of immune lymphocytes. Nature. 1970 Aug 1;227(5257):509–510. doi: 10.1038/227509a0. [DOI] [PubMed] [Google Scholar]
- Hall J. G. A method for collecting lymph from the prefemoral lymph node of unanaesthetised sheep. Q J Exp Physiol Cogn Med Sci. 1967 Apr;52(2):200–205. doi: 10.1113/expphysiol.1967.sp001902. [DOI] [PubMed] [Google Scholar]
- Hay J. B., Cahill R. N., Trnka Z. The kinetics of antigen-reactive cells during lymphocyte recruitment. Cell Immunol. 1974 Jan;10(1):145–153. doi: 10.1016/0008-8749(74)90158-0. [DOI] [PubMed] [Google Scholar]
- Hay J. B., Murphy M. J., Morris B., Bessis M. C. Quantitative studies on the proliferation and differentiation of antibody-forming cells in lymph. Am J Pathol. 1972 Jan;66(1):1–24. [PMC free article] [PubMed] [Google Scholar]
- Lipinski M., Fridman W. H., Tursz T., Vincent C., Pious D., Fellous M. Absence of allogeneic restriction in human T-cell-mediated cytotoxicity to Epstein-Barr virus-infected target cells. Demonstration of an HLA-linked control at the effector level. J Exp Med. 1979 Dec 1;150(6):1310–1322. doi: 10.1084/jem.150.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas C. J., Biddison W. E., Nelson D. L., Shaw S. Killing of measles virus-infected cells by human cytotoxic T cells. Infect Immun. 1982 Oct;38(1):226–232. doi: 10.1128/iai.38.1.226-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Ting A., Zweerink H. J., Askonas B. A. HLA restriction of cell-mediated lysis of influenza virus-infected human cells. Nature. 1977 Dec 8;270(5637):524–526. doi: 10.1038/270524a0. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Morris B. The role of the lymphatic system in the rejection of homografts: a study of lymph from renal transplants. J Exp Med. 1970 May 1;131(5):936–969. doi: 10.1084/jem.131.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K., Stroehmann I., Brandis H. Human T-cell cultures from virus-sensitized donors can mediate virus-specific and HLA-restricted cell lysis. Nature. 1980 Aug 14;286(5774):718–720. doi: 10.1038/286718a0. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Morris B. The response of the popliteal lymph node of the sheep to swine influenza virus. Aust J Exp Biol Med Sci. 1970 Feb;48(1):33–46. doi: 10.1038/icb.1970.4. [DOI] [PubMed] [Google Scholar]
- Trnka Z., Cahill R. N. Aspects of the immune response in single lymph nodes. Monogr Allergy. 1980;16:245–259. [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A., Jensen F. C. Cell-mediated immune response to lymphocytic choriomeningitis and vaccinia virus in rats. J Immunol. 1977 Oct;119(4):1242–1247. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Major transplantation antigens, viruses, and specificity of surveillance T cells. Contemp Top Immunobiol. 1977;7:179–220. doi: 10.1007/978-1-4684-3054-7_5. [DOI] [PubMed] [Google Scholar]


